Central α2-adrenergic mechanisms regulate human sympathetic neuronal discharge strategies
- PMID: 39058701
- PMCID: PMC11326960
- DOI: 10.1113/JP286450
Central α2-adrenergic mechanisms regulate human sympathetic neuronal discharge strategies
Abstract
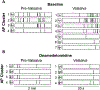
The present study investigated the impact of central α2-adrenergic mechanisms on sympathetic action potential (AP) discharge, recruitment and latency strategies. We used the microneurographic technique to record muscle sympathetic nerve activity and a continuous wavelet transform to investigate postganglionic sympathetic AP firing during a baseline condition and an infusion of a α2-adrenergic receptor agonist, dexmedetomidine (10 min loading infusion of 0.225 µg kg-1; maintenance infusion of 0.1-0.5 µg kg h-1) in eight healthy individuals (28 ± 7 years, five females). Dexmedetomidine reduced mean pressure (92 ± 7 to 80 ± 8 mmHg, P < 0.001) but did not alter heart rate (61 ± 13 to 60 ± 14 bpm; P = 0.748). Dexmedetomidine reduced sympathetic AP discharge (126 ± 73 to 27 ± 24 AP 100 beats-1, P = 0.003) most strongly for medium-sized APs (normalized cluster 2: 21 ± 10 to 5 ± 5 AP 100 beats-1; P < 0.001). Dexmedetomidine progressively de-recruited sympathetic APs beginning with the largest AP clusters (12 ± 3 to 7 ± 2 clusters, P = 0.002). Despite de-recruiting large AP clusters with shorter latencies, dexmedetomidine reduced AP latency across remaining clusters (1.18 ± 0.12 to 1.13 ± 0.13 s, P = 0.002). A subset of six participants performed a Valsalva manoeuvre (20 s, 40 mmHg) during baseline and the dexmedetomidine infusion. Compared to baseline, AP discharge (Δ 361 ± 292 to Δ 113 ± 155 AP 100 beats-1, P = 0.011) and AP cluster recruitment elicited by the Valsalva manoeuvre were lower during dexmedetomidine (Δ 2 ± 1 to Δ 0 ± 2 AP clusters, P = 0.041). The reduction in sympathetic AP latency elicited by the Valsalva manoeuvre was not affected by dexmedetomidine (Δ -0.09 ± 0.07 to Δ -0.07 ± 0.14 s, P = 0.606). Dexmedetomidine reduced baroreflex gain, most strongly for medium-sized APs (normalized cluster 2: -6.0 ± 5 to -1.6 ± 2 % mmHg-1; P = 0.008). These data suggest that α2-adrenergic mechanisms within the central nervous system modulate sympathetic postganglionic neuronal discharge, recruitment and latency strategies in humans. KEY POINTS: Sympathetic postganglionic neuronal subpopulations innervating the human circulation exhibit complex patterns of discharge, recruitment and latency. However, the central neural mechanisms governing sympathetic postganglionic discharge remain unclear. This microneurographic study investigated the impact of a dexmedetomidine infusion (α2-adrenergic receptor agonist) on muscle sympathetic postganglionic action potential (AP) discharge, recruitment and latency patterns. Dexmedetomidine infusion inhibited the recruitment of large and fast conducting sympathetic APs and attenuated the discharge of medium sized sympathetic APs that fired during resting conditions and the Valsalva manoeuvre. Dexmedetomidine infusion elicited shorter sympathetic AP latencies during resting conditions but did not affect the reductions in latency that occurred during the Valsalva manoeuvre. These data suggest that α2-adrenergic mechanisms within the central nervous system modulate sympathetic postganglionic neuronal discharge, recruitment and latency strategies in humans.
Keywords: action potential; dexmedetomidine; human; microneurography; muscle sympathetic nerve activity; valsalva manoeuvre; α2‐adrenergic receptors.
© 2024 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
Figures



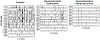

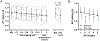

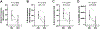



References
-
- Aars H (1972). Effects of clonidine on aortic diameter and aortic baroreceptor activity. Eur J Pharmacol 20, 52–59. - PubMed
-
- Allen AM & Guyenet PG (1993). Alpha 2-adrenoceptor-mediated inhibition of bulbospinal barosensitive cells of rat rostral medulla. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 265, R1065–R1075. - PubMed
-
- Badrov MB, Lalande S, Olver TD, Suskin N & Shoemaker JK (2016a). Effects of aging and coronary artery disease on sympathetic neural recruitment strategies during end-inspiratory and end-expiratory apnea. American Journal of Physiology-Heart and Circulatory Physiology 311, H1040–H1050. - PubMed
-
- Badrov MB, Olver TD & Shoemaker JK (2016b). Central vs. peripheral determinants of sympathetic neural recruitment: insights from static handgrip exercise and postexercise circulatory occlusion. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 311, R1013–R1021. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- F32 HL154320/HL/NHLBI NIH HHS/United States
- AHA15SDG2508009/American Heart Association (AHA)
- 00320/Canadian Government | Natural Sciences and Engineering Research Council of Canada (NSERC)
- R35 HL139854/HL/NHLBI NIH HHS/United States
- T32 DK007352/DK/NIDDK NIH HHS/United States
- T32-DK-007352/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- F32-HL-154320/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- 217916/Canadian Government | Natural Sciences and Engineering Research Council of Canada (NSERC)
- 05293/Canadian Government | Natural Sciences and Engineering Research Council of Canada (NSERC)
- K01-HL-148144/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- K01 HL148144/HL/NHLBI NIH HHS/United States
- R-35-HL-139854/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
LinkOut - more resources
Full Text Sources
Miscellaneous

