A phase 2, open-label study of anti-inflammatory NE3107 in patients with dementias
- PMID: 39058809
- PMCID: PMC11272329
- DOI: 10.1097/MD.0000000000039027
A phase 2, open-label study of anti-inflammatory NE3107 in patients with dementias
Abstract
Background: Alzheimer's disease (AD) is a progressive, multifactorial, neurodegenerative disorder affecting >6 million Americans. Chronic, low-grade neuroinflammation, and insulin resistance may drive AD pathogenesis. We explored the neurophysiological and neuropsychological effects of NE3107, an oral, anti-inflammatory, insulin-sensitizing molecule, in AD.
Methods: In this phase 2, open-label study, 23 patients with mild cognitive impairment or mild dementia received 20-mg oral NE3107 twice daily for 3 months. Primary endpoints assessed changes from baseline in neurophysiological health and oxidative stress (glutathione level) using advanced neuroimaging analyses. Secondary endpoints evaluated changes from baseline in neuropsychological health using cognitive assessments, including the 11-item Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog11), Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment, Clinical Dementia Rating, Quick Dementia Rating Scale, Alzheimer's Disease Composite Score, and Global Rating of Change (GRC). Exploratory endpoints assessed changes from baseline in neuroinflammation biomarkers (tumor necrosis factor alpha, TNF-α) and AD (amyloid beta and phosphorylated tau [P-tau]).
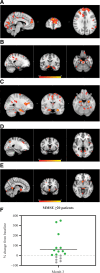
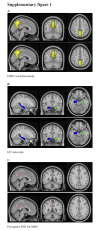
Results: NE3107 was associated with clinician-rated improvements in cerebral blood flow and functional connectivity within the brain. In patients with MMSE ≥ 20 (mild cognitive impairment to mild AD; n = 17), NE3107 was associated with directional, but statistically nonsignificant, changes in brain glutathione levels, along with statistically significant improvements in ADAS-Cog11 (P = .017), Clinical Dementia Rating (P = .042), Quick Dementia Rating Scale (P = .002), Alzheimer's Disease Composite Score (P = .0094), and clinician-rated GRC (P < .001), as well as in cerebrospinal fluid P-tau levels (P = .034) and P-tau:amyloid beta 42 ratio (P = .04). Biomarker analyses also demonstrated directional, but statistically non-significant, changes in plasma TNF-α, consistent with the expected mechanism of NE3107. Importantly, we observed a statistically significant correlation (r = 0.59) between improvements in TNF-α levels and ADAS-Cog11 scores (P = .026) in patients with baseline MMSE ≥ 20.
Conclusion: Our results indicate that in this study NE3107 was associated with what appear to be positive neurophysiological and neuropsychological findings, as well as evidence of improvement in biomarkers associated with neuroinflammation and AD in patients diagnosed with dementia. Our findings are consistent with previous preclinical and clinical observations and highlight a central role of neuroinflammation in AD pathogenesis.
Copyright © 2024 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
As employees of The Regenesis Group, Mr. Haroon, Ms. Jordan, Ms. Mahdavi, Ms. Rindner, Mr. Becerra, Mr. Surya, and Dr. Jordan report receiving grant funding from BioVie Inc. for this study. Mr. Becerra is CTO at Synaptec Network. Dr. Goodenowe has been paid fees for processing blood samples. Ms. Hofmeister’s institution has received partial grant support from Dr. Jordan. Dr. Kuhn, through Kuhn Consulting LLC, has received consulting fees from Synaptec Network, Synaptec Research, and The Regenesis Project. Dr. Jordan is CEO and founder of Synaptec Network and was responsible for processing of fMRIs on this study. Ms. Zielinski, Ms. Venkatraman, and Dr. Pourat have nothing to disclose. Dr. Zhang has received consulting fees from BioVie Inc. to prepare the data analysis of the manuscript and has stock or stock options with BioVie Inc. Mr. Ahlem and Dr. Reading are employees, stockholders, and have stock options with BioVie Inc. Dr. Palumbo is an employee and stockholder at BioVie Inc.; he has multiple patents filed as a full time compensated employee: formerly—Johnson & Johnson, Mitsubishi Tanabe, and Zynerba Pharmaceuticals; currently—BioVie Inc.; he is also an unpaid voluntary member of Dean’s Advisory Council of the George Washington University School of Medicine and Health Sciences, Washington, DC.
Figures


References
-
- Reading CL, Ahlem CN, Murphy MF. NM101 Phase III study of NE3107 in Alzheimer’s disease: rationale, design and therapeutic modulation of neuroinflammation and insulin resistance. Neurodegener Dis Manag. 2021;11:289–98. - PubMed

