Molecular mechanisms promoting long-term cytopenia after BCMA CAR-T therapy in multiple myeloma
- PMID: 39058976
- PMCID: PMC11532743
- DOI: 10.1182/bloodadvances.2023012522
Molecular mechanisms promoting long-term cytopenia after BCMA CAR-T therapy in multiple myeloma
Abstract
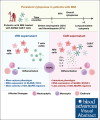
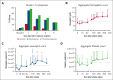
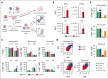
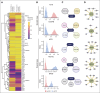
Hematologic toxicity is a common side effect of chimeric antigen receptor T-cell (CAR-T) therapies, being particularly severe among patients with relapsed or refractory multiple myeloma (MM). In this study, we characterized 48 patients treated with B-cell maturation antigen (BCMA) CAR-T cells to understand kinetics of cytopenia, identify predictive factors, and determine potential mechanisms underlying these toxicities. We observed that overall incidence of cytopenia was 95.7%, and grade >3 thrombocytopenia and neutropenia, 1 month after infusion, was observed in 57% and 53% of the patients, respectively, being still present after 1 year in 4 and 3 patients, respectively. Baseline cytopenia and high peak inflammatory markers were highly correlated with cytopenia that persisted up to 3 months. To determine potential mechanisms underlying cytopenias, we evaluated the paracrine effect of BCMA CAR-T cells on hematopoietic stem and progenitor cell (HSPC) differentiation using an ex vivo myeloid differentiation model. Phenotypic analysis showed that supernatants from activated CAR-T cells (spCAR) halted HSPC differentiation, promoting more immature phenotypes, which could be prevented with a combination of interferon γ, tumor necrosis factor α/β, transforming growth factor β, interleukin-6 (IL-6) and IL-17 inhibitors. Single-cell RNA sequencing demonstrated upregulation of transcription factors associated with early stages of hematopoietic differentiation in the presence of spCAR (GATA2, RUNX1, CEBPA) and a decrease in the activity of key regulons involved in neutrophil and monocytic maturation (ID2 and MAFB). These results suggest that CAR-T activation induces HSPC maturation arrest through paracrine effects and provides potential treatments to mitigate the severity of this toxicity.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.L.P.-B. is Hematology Program, Hospital 12 de Octubre, Madrid, Spain.
Figures

References
-
- Mikhael J, Noonan KR, Faiman B, et al. Treatment options for triple-class refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2020;20(6):351–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

