Intestinal epithelium dysfunctions cause IgA deposition in the kidney glomeruli of intestine-specific Ap1m2-deficient mice
- PMID: 39059316
- PMCID: PMC11338063
- DOI: 10.1016/j.ebiom.2024.105256
Intestinal epithelium dysfunctions cause IgA deposition in the kidney glomeruli of intestine-specific Ap1m2-deficient mice
Abstract
Background: Intestinal epithelial cells (IECs) serve as robust barriers against potentially hostile luminal antigens and commensal microbiota. Epithelial barrier dysfunction enhances intestinal permeability, leading to leaky gut syndrome (LGS) associated with autoimmune and chronic inflammatory disorders. However, a causal relationship between LGS and systemic disorders remains unclear. Ap1m2 encodes clathrin adaptor protein complex 1 subunit mu 2, which facilitates polarized protein trafficking toward the basolateral membrane and contributes to the establishment of epithelial barrier functions.
Methods: We generated IEC-specific Ap1m2-deficient (Ap1m2ΔIEC) mice with low intestinal barrier integrity as an LSG model and examined the systemic impact.
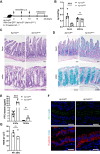
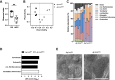
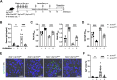
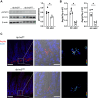
Findings: Ap1m2ΔIEC mice spontaneously developed IgA nephropathy (IgAN)-like features characterized by the deposition of IgA-IgG immune complexes and complement factors in the kidney glomeruli. Ap1m2 deficiency markedly enhanced aberrantly glycosylated IgA in the serum owing to downregulation and mis-sorting of polymeric immunoglobulin receptors in IECs. Furthermore, Ap1m2 deficiency caused intestinal dysbiosis by attenuating IL-22-STAT3 signaling. Intestinal dysbiosis contributed to the pathogenesis of IgAN because antibiotic treatment reduced aberrantly glycosylated IgA production and renal IgA deposition in Ap1m2ΔIEC mice.
Interpretation: IEC barrier dysfunction and subsequent dysbiosis by AP-1B deficiency provoke IgA deposition in the mouse kidney. Our findings provide experimental evidence of a pathological link between LGS and IgAN.
Funding: AMED, AMED-CREST, JSPS Grants-in-Aid for Scientific Research, JST CREST, Fuji Foundation for Protein Research, and Keio University Program for the Advancement of Next Generation Research Projects.
Keywords: Ap1m2; Epithelial barrier; IgA nephropathy; Intestinal microbiota; Leaky gut syndrome.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have declared that no conflict of interest exists.
Figures



References
-
- Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. - PubMed
-
- Bevins C.L., Salzman N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. - PubMed
-
- Rojas R., Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol. 2002;3:944–956. - PubMed
-
- Wei M., Shinkura R., Doi Y., Maruya M., Fagarasan S., Honjo T. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nat Immunol. 2011;12:264–270. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

