Binary fabrication of decellularized lung extracellular matrix hybridgels for in vitro chronic obstructive pulmonary disease modeling
- PMID: 39059731
- PMCID: PMC11474825
- DOI: 10.1016/j.actbio.2024.07.014
Binary fabrication of decellularized lung extracellular matrix hybridgels for in vitro chronic obstructive pulmonary disease modeling
Abstract
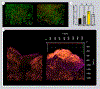
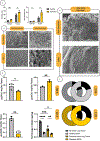
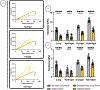
Limited treatments and a lack of appropriate animal models have spurred the study of scaffolds to mimic lung disease in vitro. Decellularized human lung and its application in extracellular matrix (ECM) hydrogels has advanced the development of these lung ECM models. Controlling the biochemical and mechanical properties of decellularized ECM hydrogels continues to be of interest due to inherent discrepancies of hydrogels when compared to their source tissue. To optimize the physiologic relevance of ECM hydrogel lung models without sacrificing the native composition we engineered a binary fabrication system to produce a Hybridgel composed of an ECM hydrogel reinforced with an ECM cryogel. Further, we compared the effect of ECM-altering disease on the properties of the gels using elastin poor Chronic Obstructive Pulmonary Disease (COPD) vs non-diseased (ND) human lung source tissue. Nanoindentation confirmed the significant loss of elasticity in hydrogels compared to that of ND human lung and further demonstrated the recovery of elastic moduli in ECM cryogels and Hybridgels. These findings were supported by similar observations in diseased tissue and gels. Successful cell encapsulation, distribution, cytotoxicity, and infiltration were observed and characterized via confocal microscopy. Cells were uniformly distributed throughout the Hybridgel and capable of survival for 7 days. Cell-laden ECM hybridgels were found to have elasticity similar to that of ND human lung. Compositional investigation into diseased and ND gels indicated the conservation of disease-specific elastin to collagen ratios. In brief, we have engineered a composited ECM hybridgel for the 3D study of cell-matrix interactions of varying lung disease states that optimizes the application of decellularized lung ECM materials to more closely mimic the human lung while conserving the compositional bioactivity of the native ECM. STATEMENT OF SIGNIFICANCE: The lack of an appropriate disease model for the study of chronic lung diseases continues to severely inhibit the advancement of treatments and preventions of these otherwise fatal illnesses due to the inability to recapture the biocomplexity of pathologic cell-ECM interactions. Engineering biomaterials that utilize decellularized lungs offers an opportunity to deconstruct, understand, and rebuild models that highlight and investigate how disease specific characteristics of the extracellular environment are involved in driving disease progression. We have advanced this space by designing a binary fabrication system for a ECM Hybridgel that retains properties from its source material required to observe native matrix interactions. This design simulates a 3D lung environment that is both mechanically elastic and compositionally relevant when derived from non-diseased tissue and pathologically diminished both mechanically and compositionally when derived from COPD tissue. Here we describe the ECM hybridgel as a model for the study of cell-ECM interactions involved in COPD.
Keywords: 3D disease modeling; Chronic obstructive pulmonary disease; Extracellular matrix; Lung disease; Tissue engineering.
Copyright © 2024 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interests The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Rebecca L. Heie reports financial support was provided by National Institutes of Health. Dr. Heise has no other competing financial interests or personal relationships to disclose. Authors Antczak, Hendrick, and Moore state that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
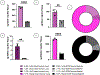




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

