Efficacy and safety of upadacitinib in patients with rheumatoid arthritis and inadequate response or intolerance to biological treatments: results through 5 years from the SELECT-BEYOND study
- PMID: 39059811
- PMCID: PMC11284939
- DOI: 10.1136/rmdopen-2023-003918
Efficacy and safety of upadacitinib in patients with rheumatoid arthritis and inadequate response or intolerance to biological treatments: results through 5 years from the SELECT-BEYOND study
Abstract
Objective: To evaluate the efficacy and safety of upadacitinib over 5 years among patients with rheumatoid arthritis (RA) in a long-term extension (LTE) of the SELECT-BEYOND phase 3 trial.
Methods: Patients refractory to ≥1 biological disease-modifying antirheumatic drug (DMARD) received upadacitinib 15 mg or 30 mg once daily or placebo, in combination with background conventional synthetic DMARD(s). At week 12, patients randomised to placebo were switched to upadacitinib 15 mg or 30 mg. All patients who completed the week 24 visit could enter the LTE for up to 5 years. Efficacy was analysed as observed and by non-responder imputation through week 260. Treatment-emergent adverse events per 100 patient-years were summarised over 5 years.
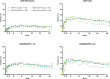
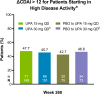
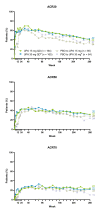
Results: Of the 498 patients randomised, 418 (84%) completed week 24 and entered the LTE. Of those who remained in the trial (n=80, upadacitinib 15 mg; n=81, upadacitinib 30 mg), 36%/36% and 81%/77% randomised to upadacitinib 15/30 mg were in Clinical Disease Activity Index (CDAI) remission or low disease activity at week 260, respectively (as observed). Approximately 47% of all patients who began in high disease activity demonstrated a CDAI improvement >12 at week 260 with upadacitinib 15/30 mg. Functional and pain-related outcomes also showed comparable improvements with both doses. Numerically higher rates of anaemia, herpes zoster and creatine phosphokinase elevation were observed with upadacitinib 30 mg vs 15 mg. No new safety issues were identified.
Conclusions: Upadacitinib 15/30 mg continued to be effective in treating clinical and functional outcomes in patients with RA. The safety profile observed over 5 years was consistent with earlier study-specific and integrated assessments of upadacitinib treatment.
Keywords: Antirheumatic Agents; Rheumatoid Arthritis; Therapeutics.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RF: research grants and consulting fees from AbbVie, Amgen, AstraZeneca, Biogen, BMS, Boehringer-Ingleheim, Flexion, Galapagos, Galvani, Genentech, Gilead, GSK, Janssen, Lilly, Novartis, Pfizer, Priovant, Roche, Sanofi-Aventis and UCB. CC-S: research grants from AbbVie, BMS, CSL Behring, Priovant Therapeutics, Alexion and Pfizer; consultant for AbbVie, Priovant Therapeutics, Octapharma, BMS, Recludix, Pfizer, Gilead and Regeneron-Sanofi. BC: consulting fees from AbbVie, BMS, Celltrion, Chugai, Gilead, Galapagos, Janssen, Lilly, MSD, Nordic, Pfizer. SH: research grants and consultancy fees from AbbVie, BMS, Lilly, Janssen, Pfizer, UCB and Novartis. AR-R: consulting fees from AbbVie, Gilead, Lilly, BMS and Sanofi; honoraria from AbbVie, Pfizer, Sanofi, UCB, BMS, Lilly, Gilead and Roche; payment for expert testimony from AbbVie and Gilead; support for travel or meeting attendance from Sanofi, Roche, Janssen, Pfizer and AbbVie; and compensation for participation on a data safety monitoring board from R-Pharm. HSC, NK, KMC and SM: employees of AbbVie and may hold stock or options.
Figures



References
-
- Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391:2513–24. doi: 10.1016/S0140-6736(18)31116-4. - DOI - PubMed
-
- Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391:2503–12. doi: 10.1016/S0140-6736(18)31115-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
