Benralizumab for allergic asthma: a randomised, double-blind, placebo-controlled trial
- PMID: 39060015
- PMCID: PMC11391094
- DOI: 10.1183/13993003.00512-2024
Benralizumab for allergic asthma: a randomised, double-blind, placebo-controlled trial
Abstract
Background: Benralizumab induces rapid and near-complete depletion of eosinophils from blood and lung tissue. We investigated whether benralizumab could attenuate the allergen-induced late asthmatic response (LAR) in participants with allergic asthma.
Methods: Participants with allergic asthma who demonstrated increased sputum eosinophils and LAR at screening were randomised to benralizumab 30 mg or matched placebo given every 4 weeks for 8 weeks (3 doses). Allergen challenges were performed at weeks 9 and 12 when blood, sputum, bone marrow and bronchial tissue eosinophils and LAR were assessed.
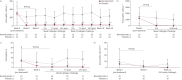
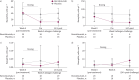
Results: 46 participants (mean age 30.9 years) were randomised to benralizumab (n=23) or placebo (n=23). Eosinophils were significantly reduced in the benralizumab group compared with placebo in blood at 4 weeks and sputum and bone marrow at 9 weeks after treatment initiation. At 7 h after an allergen challenge at week 9, sputum eosinophilia was significantly attenuated in the benralizumab group compared to placebo (least squares mean difference -5.81%, 95% CI -10.69- -0.94%; p=0.021); however, the LAR was not significantly different (least squares mean difference 2.54%, 95% CI 3.05-8.12%; p=0.363). Adverse events were reported for seven (30.4%) and 14 (60.9%) participants in the benralizumab and placebo groups, respectively.
Conclusion: Benralizumab administration over 8 weeks resulted in a significant attenuation of blood, bone marrow and sputum eosinophilia in participants with mild allergic asthma; however, there was no change in the LAR, suggesting that eosinophils alone are not a key component of allergen-induced bronchoconstriction.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: G.M. Gauvreau reports support for the present manuscript from AstraZeneca, grants from AstraZeneca, payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, and receipt of equipment, materials, drugs, medical writing, gifts or other services from AstraZeneca. R. Sehmi reports support for the present study from AstraZeneca. R. Leigh reports support for the present study from AstraZeneca, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca. D.W. Cockcroft reports support for the present manuscript from AstraZeneca, and grants from SHRF, Biohaven, AllerGen, University of Saskatchewan College of Medicine and CIHR. I. Mayers reports grants from AstraZeneca Canada and Boehringer Ingelheim, consultancy fees from Sanofi Canada and AstraZeneca, payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, and payment for expert testimony from Alberta Justice. L-P. Boulet reports grants from Amgen, AstraZeneca, GlaxoSmithKline, Merck, Novartis and Sanofi-Regeneron, royalties or licences from UptoDate and Taylor & Francis, consultancy fees from AstraZeneca, Novartis, GlaxoSmithKline, Merck and Sanofi-Regeneron, payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline, Novartis, Merck and Sanofi, and leadership role as Past-Chair of the Global Initiative for Asthma (GINA) Board of Directors, Past President of the Global Asthma Organisation (Interasma), Past Member of the Canadian Thoracic Society Respiratory Guidelines Committee and Past Laval University Chair on Knowledge Transfer, Prevention and Education in Respiratory and Cardiovascular Health. T. Ho reports grants from Fisher & Paykel, consultancy fees from Valeo and AstraZeneca, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca and GlaxoSmithKline. I. Satia reports grants from Merck, GlaxoSmithKline and Bellus, consultancy fees from Merck, Genentech, Respiplus and GlaxoSmithKline/Bellus, and payment or honoraria for lectures, presentations, manuscript writing or educational events from Merck, GlaxoSmithKline, AstraZeneca and Sanofi. P.D. Mitchell reports grants from Teva, consultancy fees from Pfizer and GlaxoSmithKline, and support for attending meetings from AstraZeneca. I.P. Magee reports payment or honoraria for lectures, presentations, manuscript writing or educational events from GlaxoSmithKline. C. Bergeron reports support for the present study from AstraZeneca, grants from AstraZeneca, Sanofi and Regeneron, consultancy fees from ValeoPharma, Sanofi, AstraZeneca and GlaxoSmithKline, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline, ValeoPharma, Sanofi and Grifols. M. Bhutani reports grants from CIHR, GlaxoSmithKline, AstraZeneca and Sanofi, consultancy fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Sanofi, Covis and Valeo, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline, Sanofi and Covis. V. Werkström was an employee of AstraZeneca at the time of the study and reports stock or stock options in AstraZeneca. T. Durżyński was an employee of AstraZeneca at the time of the study and reports stock or stock options in AstraZeneca. K. Shoemaker was an employee of AstraZeneca at the time of the study and reports stock or stock options in AstraZeneca. R.K. Katial was an employee of AstraZeneca at the time of the study and reports personal fees from AstraZeneca. M. Jison was an employee of AstraZeneca at the time of the study and reports stock or stock options in AstraZeneca. C. McCrae was an employee of AstraZeneca at the time of the study and reports stock or stock options in AstraZeneca. P.M. O'Byrne reports support for the present study from AstraZeneca, grants from AstraZeneca, Merck and Biohaven, consultancy fees from AstraZeneca, GlaxoSmithKline, Sage, Teva and Affibody, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Chiesi, GlaxoSmithKline and Covis. The remaining authors have no potential conflicts of interest to disclose.
Figures


Comment in
-
Allergen challenge, eosinophils and the long road to asthma endotypes.Eur Respir J. 2024 Sep 12;64(3):2401316. doi: 10.1183/13993003.01316-2024. Print 2024 Sep. Eur Respir J. 2024. PMID: 39266229 No abstract available.
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for the Diagnosis, Management and Prevention of COPD. 2022. Available from: http://goldcopd.org/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
