Targeting a STING agonist to perivascular macrophages in prostate tumors delays resistance to androgen deprivation therapy
- PMID: 39060021
- PMCID: PMC11284826
- DOI: 10.1136/jitc-2024-009368
Targeting a STING agonist to perivascular macrophages in prostate tumors delays resistance to androgen deprivation therapy
Abstract
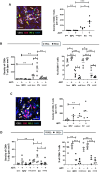
Background: Androgen deprivation therapy (ADT) is a front-line treatment for prostate cancer. In some men, their tumors can become refractory leading to the development of castration-resistant prostate cancer (CRPC). This causes tumors to regrow and metastasize, despite ongoing treatment, and impacts negatively on patient survival. ADT is known to stimulate the accumulation of immunosuppressive cells like protumoral tumor-associated macrophages (TAMs), myeloid-derived suppressor cells and regulatory T cells in prostate tumors, as well as hypofunctional T cells. Protumoral TAMs have been shown to accumulate around tumor blood vessels during chemotherapy and radiotherapy in other forms of cancer, where they drive tumor relapse. Our aim was to see whether such perivascular (PV) TAMs also accumulate in ADT-treated prostate tumors prior to CRPC, and, if so, whether selectively inducing them to express a potent immunostimulant, interferon beta (IFNβ), would stimulate antitumor immunity and delay CRPC.
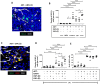
Methods: We used multiplex immunofluorescence to assess the effects of ADT on the distribution and activation status of TAMs, CD8+T cells, CD4+T cells and NK cells in mouse and/or human prostate tumors. We then used antibody-coated, lipid nanoparticles (LNPs) to selectively target a STING agonist, 2'3'-cGAMP (cGAMP), to PV TAMs in mouse prostate tumors during ADT.
Results: TAMs accumulated at high density around blood vessels in response to ADT and expressed markers of a protumoral phenotype including folate receptor-beta (FR-β), MRC1 (CD206), CD169 and VISTA. Additionally, higher numbers of inactive (PD-1-) CD8+T cells and reduced numbers of active (CD69+) NK cells were present in these PV tumor areas. LNPs coated with an antibody to FR-β selectively delivered cGAMP to PV TAMs in ADT-treated tumors, where they activated STING and upregulated the expression of IFNβ. This resulted in a marked increase in the density of active CD8+T cells (along with CD4+T cells and NK cells) in PV tumor areas, and significantly delayed the onset of CRPC. Antibody depletion of CD8+T cells during LNP administration demonstrated the essential role of these cells in delay in CRPC induced by LNPs.
Conclusion: Together, our data indicate that targeting a STING agonist to PV TAMs could be used to extend the treatment window for ADT in prostate cancer.
Keywords: immunotherapy; macrophage; prostate cancer.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
