Preclinical activity of allogeneic SLAMF7-specific CAR T-cells (UCARTCS1) in multiple myeloma
- PMID: 39060023
- PMCID: PMC11284884
- DOI: 10.1136/jitc-2023-008769
Preclinical activity of allogeneic SLAMF7-specific CAR T-cells (UCARTCS1) in multiple myeloma
Abstract
Background: Autologous BCMA-specific CAR T-cell therapies have substantial activity in multiple myeloma (MM). However, due to logistical limitations and BCMAlow relapses, there is a need for alternatives. UCARTCS1 cells are 'off-the-shelf' allogeneic CAR T-cells derived from healthy donors targeting SLAMF7 (CS1), which is highly expressed in MM cells. In this study, we evaluated the preclinical activity of UCARTCS1 in MM cell lines, in bone marrow (BM) samples obtained from MM patients and in an MM mouse model.
Methods: Luciferase-transduced MM cell lines were incubated with UCARTCS1 cells or control (non-transduced, SLAMF7/TCRαβ double knock-out) T-cells at different effector to target ratios for 24 hours. MM cell lysis was assessed by bioluminescence. Anti-MM activity of UCARTCS1 was also evaluated in 29 BM samples obtained from newly diagnosed patients (n=10), daratumumab-naïve relapsed/refractory patients (n=10) and daratumumab-refractory patients (n=9) in 24-hour flow cytometry-based cytotoxicity assays. Finally, UCARTCS1 activity was assessed in mouse xenograft models.
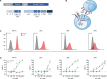
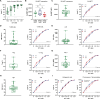
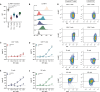
Results: UCARTCS1 cells induced potent CAR-mediated, and dose-dependent lysis of both MM cell lines and primary MM cells. There was no difference in ex vivo activity of UCARTCS1 between heavily pretreated and newly diagnosed patients. In addition, efficacy of UCARTCS1 was not affected by SLAMF7 expression level on MM cells, proportion of tumor cells, or frequency of regulatory T-cells in BM samples obtained from MM patients. UCARTCS1 treatment eliminated SLAMF7+ non-malignant immune cells in a dose-dependent manner, however lysis of normal cells was less pronounced compared to that of MM cells. Additionally, durable anti-MM responses were observed with UCARTCS1 in an MM xenograft model.
Conclusions: These results demonstrate that UCARTCS1 has potent anti-MM activity against MM cell lines and primary MM cells, as well as in an MM xenograft model and support the evaluation of UCARTCS1 in patients with advanced MM.
Keywords: Chimeric antigen receptor - CAR; Immunotherapy; Multiple Myeloma; T cell.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DLC, IC-S and RG are employees of Cellectis SA. MT has received research support from Kite-Gilead, consultancy compensation from Sangamo Therapeutics and is inventor of licensed patents and patent applications related to the development of CARs and CAR-T cells from iPSC. SZ has received research funding from Celgene, Takeda, and Janssen Pharmaceuticals and serves on advisory boards for Janssen Pharmaceuticals, Sanofi, Celgene, Takeda, Amgen and Oncopeptides. No personal funding. TM has received research support from Janssen Pharmaceuticals, Genmab, Takeda, Novartis and ONK Therapeutics. NWCJvdD has received research support from Janssen Pharmaceuticals, AMGEN, Celgene, Novartis, Cellectis and BMS and serves on advisory boards for Janssen Pharmaceuticals, AMGEN, Celgene, BMS, Takeda, Roche, Novartis, Bayer, Pfizer, Kite Pharma, Merck, Abbvie, Adaptive and Servier, all paid to employer.
Figures


References
-
- Mateos M-V, Weisel K, De Stefano V, et al. LocoMMotion: a prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed/refractory multiple myeloma (RRMM) receiving ≥3 prior lines of therapy. J Clin Oncol. 2021;39:8041. doi: 10.1200/JCO.2021.39.15_suppl.8041. - DOI - PMC - PubMed
-
- Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–24. doi: 10.1016/S0140-6736(21)00933-8. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
