Quiescence Enhances Survival during Viral Infection in Caenorhabditis elegans
- PMID: 39060176
- PMCID: PMC11358607
- DOI: 10.1523/JNEUROSCI.1700-22.2024
Quiescence Enhances Survival during Viral Infection in Caenorhabditis elegans
Abstract
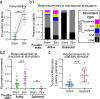
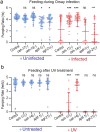
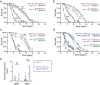
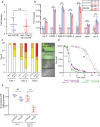
Infection causes reduced activity, anorexia, and sleep, which are components of the phylogenetically conserved but poorly understood sickness behavior. We developed a Caenorhabditis elegans model to study quiescence during chronic infection, using infection with the Orsay virus. The Orsay virus infects intestinal cells yet strongly affects behavior, indicating gut-to-nervous system communication. Infection quiescence has the sleep properties of reduced responsiveness and rapid reversibility. Both the ALA and RIS neurons regulate virus-induced quiescence though ALA plays a more prominent role. Quiescence-defective animals have decreased survival when infected, indicating a benefit of quiescence during chronic infectious disease. The survival benefit of quiescence is not explained by a difference in viral load, indicating that it improves resilience rather than resistance to infection. Orsay infection is associated with a decrease in ATP levels, and this decrease is more severe in quiescence-defective animals. We propose that quiescence preserves energetic resources by reducing energy expenditures and/or by increasing extraction of energy from nutrients. This model presents an opportunity to explore the role of sleep and fatigue in chronic infectious illness.
Keywords: C. elegans; fatigue; neuropeptides; sleep; virus.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
