Role of mucosal-associated invariant T cells dynamics in pathogenesis of Sjögren syndrome
- PMID: 39060324
- PMCID: PMC11282087
- DOI: 10.1038/s41598-024-67901-1
Role of mucosal-associated invariant T cells dynamics in pathogenesis of Sjögren syndrome
Abstract
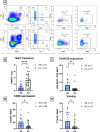
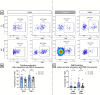
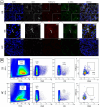
Sjögren syndrome (SS) is an autoimmune disease characterized by chronic inflammatory infiltrates in the salivary and lacrimal glands. Mucosal-associated invariant T (MAIT) cells are a subset of innate-like T-cells, predominantly found in mucosal tissues with crucial role in epithelial homeostasis. Thus, MAIT cells may be implicated in mucosal alterations of SS patients. Activation markers, inflammatory and cytotoxic cytokines were examined in 23 SS patients and compared to 23 healthy controls (HC). Tissular MAIT cells in salivary gland (SG) biopsies were also analyzed. Circulating MAIT cells were decreased in SS patients with a higher expression of CD69 and a higher CD4/CD8 ratio of MAIT cells. MAIT cells showed a higher production of IFNγ, TNFα and GzB in SS compare to HC. Tissular MAIT cells were present within inflamed SG of SS patients, while they were absent in SG of HC. Overall, circulating MAIT cells are decreased in the peripheral blood of SS albeit producing higher amounts of IFNγ, TNFα, and GzB. Tissular MAIT cells are detected in salivary glands from SS with a proinflammatory tissular cytokine environment. MAIT cells with abnormal phenotype, functions and tissular homeostasis may contribute to epithelial damage in SS.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Shiboski, C. H. et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis.76(1), 9–16 (2017). 10.1136/annrheumdis-2016-210571 - DOI - PubMed
-
- Flament, H. et al. Outcome of SARS-CoV-2 infection is linked to MAIT cell activation and cytotoxicity. Nat. Immunol.5, 1 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

