Ferroptosis is a targetable detrimental factor in metabolic dysfunction-associated steatotic liver disease
- PMID: 39060422
- PMCID: PMC11369286
- DOI: 10.1038/s41418-024-01348-9
Ferroptosis is a targetable detrimental factor in metabolic dysfunction-associated steatotic liver disease
Abstract
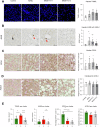
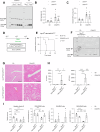
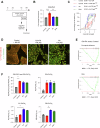
There is an unmet clinical need for pharmacologic treatment for metabolic dysfunction-associated steatotic liver disease (MASLD). Hepatocyte cell death is a hallmark of this highly prevalent chronic liver disease, but the dominant type of cell death remains uncertain. Here we report that ferroptosis, an iron-catalyzed mode of regulated cell death, contributes to MASLD. Unsupervised clustering in a cohort of biopsy-proven MASLD patients revealed a subgroup with hepatic ferroptosis signature and lower glutathione peroxidase 4 (GPX4) levels. Likewise, a subgroup with reduced ferroptosis defenses was discerned in public transcriptomics datasets. Four weeks of choline-deficient L-amino acid-defined high-fat diet (CDAHFD) induced MASLD with ferroptosis in mice. Gpx4 overexpression did not affect steatohepatitis, instead CDAHFD protected from morbidity due to hepatocyte-specific Gpx4 knockout. The ferroptosis inhibitor UAMC-3203 attenuated steatosis and alanine aminotransferase in CDAHFD and a second model, i.e., the high-fat high-fructose diet (HFHFD). The effect of monounsaturated and saturated fatty acids supplementation on ferroptosis susceptibility was assessed in human HepG2 cells. Fat-laden HepG2 showed a drop in ferroptosis defenses, increased phosphatidylglycerol with two polyunsaturated fatty acid (PUFA) lipid tails, and sustained ferroptosis sensitivity. In conclusion, this study identified hepatic ferroptosis as a detrimental factor in MASLD patients. Unexpectedly, non-PUFA supplementation to hepatocytes altered lipid bilayer composition to maintain ferroptosis sensitivity. Based on findings in in vivo models, ferroptosis inhibition represents a promising therapeutic target in MASLD.
© 2024. The Author(s).
Conflict of interest statement
TVB and KA own patents related to ferroptosis inhibitors (US9862678, WO2016075330, EP3218357, WO2019154795). All other authors declare that they have no financial or non-financial interests related to this manuscript. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

