Combination of PARP Inhibitors and Androgen Receptor Pathway Inhibitors in Metastatic Castration-Resistant Prostate Cancer
- PMID: 39060912
- PMCID: PMC11438617
- DOI: 10.1007/s40265-024-02071-y
Combination of PARP Inhibitors and Androgen Receptor Pathway Inhibitors in Metastatic Castration-Resistant Prostate Cancer
Abstract
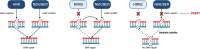
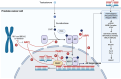
Despite recent advances in the treatment of metastatic prostate cancer, progression to a castration-resistant state remains inevitable for most and prognosis is limited. Genetic testing for homologous recombination repair pathway alterations is recommended for all patients with advanced prostate cancer given that a mutation is present in up to 25% of cases. Poly(ADP-ribose) polymerase (PARPis) are now approved for use in patients with metastatic castration-resistant prostate cancer who have progressed on an androgen receptor pathway inhibitor (ARPI) and harbour a germline or somatic homologous recombination repair mutation. Preclinical data support a synergistic effect with an ARPI and PARPi, and various ARPI-PARPi combinations have therefore been explored in phase III clinical trials. Despite heterogeneous findings, a clear hierarchy of benefit is evident, with patients harbouring a BRCA mutation deriving the greatest magnitude of benefit, followed by any homologous recombination repair mutation. The benefit in homologous recombination repair-proficient cohort is less clear, and questions remain about whether ARPI-PARPi combination therapy should be offered to patients without a homologous recombination repair mutation. With ARPIs now considered standard-of-care for metastatic hormone-sensitive prostate cancer, ARPI-PARPi combination therapy is currently being explored earlier in the treatment paradigm. The purpose of this review is to discuss the rationale behind ARPI-PARPi combination therapy, summarise the results of key clinical trials, and discuss clinical considerations and future perspectives.
© 2024. The Author(s).
Conflict of interest statement
Louise Kostos has received honoraria for speaking duties from Bayer and Astra Zeneca. Ben Tran declares grants and personal fees from Amgen, Astra Zeneca, Astellas, Bristol Myers Squibb, Merck Sharpe Dome, Merck Serono, Pfizer, Janssen, Bayer, Ipsen, Roche and Sanofi. Arun A. Azad declares the following lifetime disclosures: consultant: Aculeus Therapeutics, Astellas, Janssen, Novartis. Honoraria: Aculeus Therapeutics, Amgen, Arvinas, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Ipsen, Janssen, Merck Serono, Merck Sharpe Dohme, Novartis, Noxopharm, Pfizer, Sanofi, Telix, Tolmar. Scientific advisory board: Amgen, Arvinas, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Ipsen, Janssen, Merck Serono, Merck Sharpe Dohme, Novartis, Noxopharm, Pfizer, Sanofi, Telix, Tolmar. Travel and accommodation: Amgen, Astellas, Bayer, Hinova, Janssen, Merck Serono, Novartis, Pfizer, Tolmar. Research funding: Aptevo Therapeutics (institutional), Astellas (investigator), Astellas (institutional), Astra Zeneca (institutional), Astra Zeneca (investigator), Bionomics (institutional), Bristol Myers Squibb (institutional), Eli Lilly (institutional), Exelixis (institutional), Gilead Sciences (institutional), Glaxo Smith Kline (institutional), Hinova (institutional), Ipsen (institutional), Janssen (institutional), MedImmune (institutional), Merck Serono (investigator), Merck Serono (institutional), Merck Sharpe Dohme (institutional), Novartis (institutional), Pfizer (institutional), Sanofi Aventis (institutional), SYNthorx (institutional). Steering Committee Member: Astra Zeneca, Arvinas, Astellas, Exelixis, Janssen and Pfizer.
Figures
Similar articles
-
Efficacy of Poly(ADP-ribose) Polymerase Inhibitors by Individual Genes in Homologous Recombination Repair Gene-Mutated Metastatic Castration-Resistant Prostate Cancer: A US Food and Drug Administration Pooled Analysis.J Clin Oncol. 2024 May 10;42(14):1687-1698. doi: 10.1200/JCO.23.02105. Epub 2024 Mar 14. J Clin Oncol. 2024. PMID: 38484203 Free PMC article.
-
Systemic Therapy in Patients With Metastatic Castration-Resistant Prostate Cancer: ASCO Guideline Update.J Clin Oncol. 2025 Jul 10;43(20):2311-2334. doi: 10.1200/JCO-25-00007. Epub 2025 May 2. J Clin Oncol. 2025. PMID: 40315400
-
Empowering PARP inhibition through rational combination: Mechanisms of PARP inhibitors and combinations with a focus on the treatment of metastatic castration-resistant prostate cancer.Crit Rev Oncol Hematol. 2025 Jun;210:104698. doi: 10.1016/j.critrevonc.2025.104698. Epub 2025 Mar 13. Crit Rev Oncol Hematol. 2025. PMID: 40089046 Review.
-
First-line combination treatment with PARP and androgen receptor-signaling inhibitors in HRR-deficient mCRPC: Applying clinical study findings to clinical practice in the United States.Cancer Treat Rev. 2024 May;126:102726. doi: 10.1016/j.ctrv.2024.102726. Epub 2024 Mar 29. Cancer Treat Rev. 2024. PMID: 38613872 Review.
-
Synthetic Lethality by Co-Inhibition of Androgen Receptor and Polyadenosine Diphosphate-Ribose in Metastatic Prostate Cancer.Int J Mol Sci. 2023 Dec 20;25(1):78. doi: 10.3390/ijms25010078. Int J Mol Sci. 2023. PMID: 38203248 Free PMC article. Review.
Cited by
-
Update on Combination Strategies of PARP Inhibitors.Cancer Control. 2024 Jan-Dec;31:10732748241298329. doi: 10.1177/10732748241298329. Cancer Control. 2024. PMID: 39500600 Free PMC article. Review.
-
Circulating Cell-Free DNA Integrity for Breast and Prostate Cancer: What Is the Landscape for Clinical Management of the Most Common Cancers in Women and Men?Int J Mol Sci. 2025 Jan 22;26(3):900. doi: 10.3390/ijms26030900. Int J Mol Sci. 2025. PMID: 39940669 Free PMC article. Review.
-
Prognostic Role of Prostate-specific Antigen Isoforms and Their Early Kinetics in Patients With Metastatic Castration-resistant Prostate Cancer Receiving New Generation Androgen Receptor Targeted Agents.In Vivo. 2025 Mar-Apr;39(2):859-869. doi: 10.21873/invivo.13889. In Vivo. 2025. PMID: 40010964 Free PMC article.
-
Strategic Advances in Combination Therapy for Metastatic Castration-Sensitive Prostate Cancer: Current Insights and Future Perspectives.Cancers (Basel). 2024 Sep 18;16(18):3187. doi: 10.3390/cancers16183187. Cancers (Basel). 2024. PMID: 39335158 Free PMC article. Review.
-
Innovations and Emerging Trends in Prostate Cancer Management: A Literature Review.Cureus. 2024 Nov 6;16(11):e73128. doi: 10.7759/cureus.73128. eCollection 2024 Nov. Cureus. 2024. PMID: 39512805 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

