Signaling dynamics in coexisting monoclonal cell subpopulations unveil mechanisms of resistance to anti-cancer compounds
- PMID: 39061010
- PMCID: PMC11282632
- DOI: 10.1186/s12964-024-01742-3
Signaling dynamics in coexisting monoclonal cell subpopulations unveil mechanisms of resistance to anti-cancer compounds
Abstract
Background: Tumor heterogeneity is a main contributor of resistance to anti-cancer targeted agents though it has proven difficult to study. Unfortunately, model systems to functionally characterize and mechanistically study dynamic responses to treatment across coexisting subpopulations of cancer cells remain a missing need in oncology.
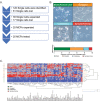
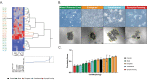
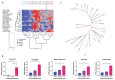
Methods: Using single cell cloning and expansion techniques, we established monoclonal cell subpopulations (MCPs) from a commercially available epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer cell line. We then used this model sensitivity to the EGFR inhibitor osimertinib across coexisting cell populations within the same tumor. Pathway-centered signaling dynamics associated with response to treatment and morphological characteristics of the MCPs were assessed using Reverse Phase Protein Microarray. Signaling nodes differentially activated in MCPs less sensitive to treatment were then pharmacologically inhibited to identify target signaling proteins putatively implicated in promoting drug resistance.
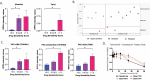
Results: MCPs demonstrated highly heterogeneous sensitivities to osimertinib. Cell viability after treatment increased > 20% compared to the parental line in selected MCPs, whereas viability decreased by 75% in other MCPs. Reduced treatment response was detected in MCPs with higher proliferation rates, EGFR L858R expression, activation of EGFR binding partners and downstream signaling molecules, and expression of epithelial-to-mesenchymal transition markers. Levels of activation of EGFR binding partners and MCPs' proliferation rates were also associated with response to c-MET and IGFR inhibitors.
Conclusions: MCPs represent a suitable model system to characterize heterogeneous biomolecular behaviors in preclinical studies and identify and functionally test biological mechanisms associated with resistance to targeted therapeutics.
© 2024. The Author(s).
Conflict of interest statement
The authors are inventors on US Government and University assigned patents and patent applications that cover aspects of the technologies. As inventors, they are entitled to receive royalties as provided by US Law and George Mason University policy. MP, VE, and EP receive royalties from TheraLink Technologies, Inc. MP, VE, and EP are consultants to and/or shareholders of TheraLink Technologies, Inc; EP is shareholder and consultant of Perthera, Inc.
Figures


Similar articles
-
PIM1 kinase promotes EMT-associated osimertinib resistance via regulating GSK3β signaling pathway in EGFR-mutant non-small cell lung cancer.Cell Death Dis. 2024 Sep 3;15(9):644. doi: 10.1038/s41419-024-07039-0. Cell Death Dis. 2024. PMID: 39227379 Free PMC article.
-
AXIN1/MYC Axis Mediated the Osimertinib Resistance in EGFR Mutant Non-Small Cell Lung Cancer Cells.Tohoku J Exp Med. 2024 May 1;262(4):269-276. doi: 10.1620/tjem.2024.J002. Epub 2024 Jan 18. Tohoku J Exp Med. 2024. PMID: 38233113
-
Trametinib overcomes KRAS-G12V-induced osimertinib resistance in a leptomeningeal carcinomatosis model of EGFR-mutant lung cancer.Cancer Sci. 2021 Sep;112(9):3784-3795. doi: 10.1111/cas.15035. Epub 2021 Jul 22. Cancer Sci. 2021. PMID: 34145930 Free PMC article.
-
Treatment of Brain Metastases of Non-Small Cell Lung Carcinoma.Int J Mol Sci. 2021 Jan 8;22(2):593. doi: 10.3390/ijms22020593. Int J Mol Sci. 2021. PMID: 33435596 Free PMC article. Review.
-
Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer.Br J Cancer. 2019 Oct;121(9):725-737. doi: 10.1038/s41416-019-0573-8. Epub 2019 Sep 30. Br J Cancer. 2019. PMID: 31564718 Free PMC article. Review.
Cited by
-
Strategies Beyond 3rd EGFR-TKI Acquired Resistance: Opportunities and Challenges.Cancer Med. 2025 May;14(9):e70921. doi: 10.1002/cam4.70921. Cancer Med. 2025. PMID: 40322930 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

