Target-Driven Tissue-Agnostic Drug Approvals-A New Path of Drug Development
- PMID: 39061168
- PMCID: PMC11274498
- DOI: 10.3390/cancers16142529
Target-Driven Tissue-Agnostic Drug Approvals-A New Path of Drug Development
Abstract
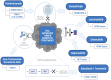
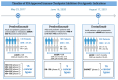
The regulatory approvals of tumor-agnostic therapies have led to the re-evaluation of the drug development process. The conventional models of drug development are histology-based. On the other hand, the tumor-agnostic drug development of a new drug (or combination) focuses on targeting a common genomic biomarker in multiple cancers, regardless of histology. The basket-like clinical trials with multiple cohorts allow clinicians to evaluate pan-cancer efficacy and toxicity. There are currently eight tumor agnostic approvals granted by the Food and Drug Administration (FDA). This includes two immune checkpoint inhibitors, and five targeted therapy agents. Pembrolizumab is an anti-programmed cell death protein-1 (PD-1) antibody that was the first FDA-approved tumor-agnostic treatment for unresectable or metastatic microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR) solid tumors in 2017. It was later approved for tumor mutational burden-high (TMB-H) solid tumors, although the TMB cut-off used is still debated. Subsequently, in 2021, another anti-PD-1 antibody, dostarlimab, was also approved for dMMR solid tumors in the refractory setting. Patients with fusion-positive cancers are typically difficult to treat due to their rare prevalence and distribution. Gene rearrangements or fusions are present in a variety of tumors. Neurotrophic tyrosine kinase (NTRK) fusions are present in a range of pediatric and adult solid tumors in varying frequency. Larotrectinib and entrectinib were approved for neurotrophic tyrosine kinase (NTRK) fusion-positive cancers. Similarly, selpercatinib was approved for rearranged during transfection (RET) fusion-positive solid tumors. The FDA approved the first combination therapy of dabrafenib, a B-Raf proto-oncogene serine/threonine kinase (BRAF) inhibitor, plus trametinib, a mitogen-activated protein kinase (MEK) inhibitor for patients 6 months or older with unresectable or metastatic tumors (except colorectal cancer) carrying a BRAFV600E mutation. The most recent FDA tumor-agnostic approval is of fam-trastuzumab deruxtecan-nxki (T-Dxd) for HER2-positive solid tumors. It is important to identify and expeditiously develop drugs that have the potential to provide clinical benefit across tumor types.
Keywords: V-Raf murine sarcoma viral oncogene homolog B V600E (BRAFV600E); dabrafenib and trametinib; dostarlimab-gxly; entrectinib; larotrectinib; microsatellite instability-high (MSI-H); neurotrophic tyrosine kinase (NTRK); pembrolizumab; precision oncology; rearranged during transfection (RET); selpercatinib; tissue-agnostic drug development; tumor mutational burden-high (TMB-H).
Conflict of interest statement
Kyaw Z. Thein received honoraria from Targeted Oncology, Curio Science, Aptitude Health, OMNI-Oncology, and Onviv Expert Network and served on the Advisory Board for EMD Serono and on the Speaker Bureau for Eisai, not related to this manuscript. All others declare no conflicts of interest.
Figures

References
-
- FDA Grants Accelerated Approval to Pembrolizumab for First Tissue/Site Agnostic Indication. [(accessed on 30 June 2024)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grant....
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

