Non-Invasive 3D Breast Tumor Localization: A Viable Alternative to Invasive Tumor Marking
- PMID: 39061203
- PMCID: PMC11274474
- DOI: 10.3390/cancers16142564
Non-Invasive 3D Breast Tumor Localization: A Viable Alternative to Invasive Tumor Marking
Abstract
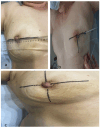
Background: We present a detailed description and the preliminary results of our original technique for non-invasive three-dimensional tumor localization in the breast, which was created as an alternative to standard invasive tumor marking before neoadjuvant systemic therapy (NAST), aiming to enable adequate surgery after complete tumor regression. Methods: A detailed description of the technique is provided in the main text. The technique's feasibility and precision were assessed in a single-arm, prospective study based on the histological parameters of the adequacy and rationality of the excision of completely regressed tumor beds. Results: Out of 94 recruited patients, 15 (16%) were deemed unsuitable, mainly due to the tumors' inadequate ultrasound visibility. Among the 79 processed patients, 31 (39%) had complete clinical regression after NAST and were operated on using our technique. The histological parameters of surgical precision (signs of tumor regression: 24/31; microscopic cancer residues: 7/31) were verified in all excised specimens (100% precision). There were no positive margins in seven cases with microscopic residues, indicating our technique's capacity to enable oncologically safe post-NAST surgery. Conclusions: The proposed technique is feasible and satisfactorily accurate in determining the location of regressed tumors, thus representing an alternative to invasive tumor marking, especially in surgical centers lacking trained staff and equipment for invasive marking. The technique's limitations are mainly related to the inadequate ultrasound visibility of the tumor.
Keywords: early breast cancer; marking techniques; neoadjuvant systemic therapy; pathological complete response; surgical excision; tumor positioning; ultrasound.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Fisher B., Bryant J., Wolmark N., Mamounas E., Brown A., Fisher E.R., Wickerham D.L., Begovic M., DeCillis A., Robidoux A., et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. - DOI - PubMed
LinkOut - more resources
Full Text Sources

