Reduced Dose of Post-Transplant Cyclophosphamide with Tacrolimus for the Prevention of Graft-versus-Host Disease in HLA-Matched Donor Peripheral Blood Stem Cell Transplants: A Prospective Pilot Study
- PMID: 39061206
- PMCID: PMC11274764
- DOI: 10.3390/cancers16142567
Reduced Dose of Post-Transplant Cyclophosphamide with Tacrolimus for the Prevention of Graft-versus-Host Disease in HLA-Matched Donor Peripheral Blood Stem Cell Transplants: A Prospective Pilot Study
Abstract
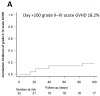
PTCY 50 mg/kg/day on days +3/+4 is an excellent strategy to prevent GVHD. However, its use is associated with adverse outcomes such as delayed engraftment, increased risk of infection, and cardiac complications. This pilot study evaluates the efficacy and toxicity of a reduced dose of PTCY (40 mg/kg/day) combined with tacrolimus in 22 peripheral blood HLA-matched alloHSCT patients. At day +100, the cumulative incidences of grade II-IV and III-IV acute GVHD were 18.2% and 4.5%, respectively. No grade IV acute GVHD or steroid-refractory disease was observed. The cumulative incidences of all-grade and moderate-severe chronic GVHD at 1-year were 11.4% and 6.4%, respectively. No patient died from transplant-related complications. Two-year OS and RFS were 77.1% and 58.3%, respectively. All patients engrafted, with neutrophil and platelet recovery occurring at a median of 15 (IQR 14-16) and 16 days (IQR 12-23), respectively. The cumulative incidences of bloodstream bacterial infections, polyomavirus BK hemorrhagic cystitis, HHV6 reactivation, CMV reactivation, and fungal infections were 13.6%, 9.1%, 9.1%, 4.6%, and 6%, respectively. Only one early cardiac event was observed. These results suggest that PTCY 40 mg/kg/day on a +3/+4 schedule provides adequate immunosuppression to allow for engraftment and prevent clinically significant GVHD with a low toxicity profile.
Keywords: GVHD; allogeneic hematopoietic stem cell transplantation; graft-versus-host disease; post-transplant cyclophosphamide; reduced-dose PTCY.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Luznik L., O’Donnell P.V., Symons H.J., Chen A.R., Leffell M.S., Zahurak M., Gooley T.A., Piantadosi S., Kaup M., Ambinder R.F., et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol. Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. - DOI - PMC - PubMed
-
- Kanakry C.G., Tsai H.L., Bolanos-Meade J., Smith B.D., Gojo I., Kanakry J.A., Kasamon Y.L., Gladstone D.E., Matsui W., Borrello I., et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. 2014;124:3817–3827. doi: 10.1182/blood-2014-07-587477. - DOI - PMC - PubMed
-
- Sanz J., Galimard J.E., Labopin M., Afanasyev B., Sergeevich M.I., Angelucci E., Kroger N., Koc Y., Ciceri F., Diez-Martin J.L., et al. Post-transplant cyclophosphamide containing regimens after matched sibling, matched unrelated and haploidentical donor transplants in patients with acute lymphoblastic leukemia in first complete remission, a comparative study of the ALWP of the EBMT. J. Hematol. Oncol. 2021;14:84. doi: 10.1186/s13045-021-01094-2. - DOI - PMC - PubMed
-
- Ruggeri A., Labopin M., Bacigalupo A., Afanasyev B., Cornelissen J.J., Elmaagacli A., Itala-Remes M., Blaise D., Meijer E., Koc Y., et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J. Hematol. Oncol. 2018;11:40. doi: 10.1186/s13045-018-0586-4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

