PD-L1 Expression in Paired Samples of Rectal Cancer
- PMID: 39061244
- PMCID: PMC11275196
- DOI: 10.3390/cancers16142606
PD-L1 Expression in Paired Samples of Rectal Cancer
Abstract
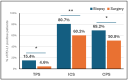
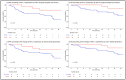
Immune checkpoint inhibitors and immune-related biomarkers are increasingly investigated in rectal cancer (RC). We retrospectively analysed PD-L1 expression in diagnostic biopsy and resection samples from RC patients treated at our centre between 2000 and 2020. PD-L1 immunostaining (22C3 clone) was evaluated according to tumour proportion (TPS), immune cell (ICS), and the combined positive score (CPS). Eighty-three patients were included. At diagnosis, PD-L1 expression ≥1%/≥5% was observed in 15.4%/0%, 80.7%/37.4%, and 69.2%/25.6% of patients based on TPS, ICS, and CPS, respectively. At surgery, the respective figures were 4.6%/1.5%, 60.2%/32.5%, and 50.7%/26.2%. Using the 1% cut-off and regardless of the scoring system, PD-L1 was less expressed in surgery than biopsy samples (p ≤ 0.04). In paired specimens, PD-L1-ICS reduction was especially observed following neoadjuvant long-course (chemo)radiotherapy (p = 0.03). PD-L1-ICS of ≥5% in surgical samples (HR: 0.17; p = 0.02), and a biopsy-to-surgery increase in PD-L1-ICS (HR: 0.19; p = 0.04) was predictive for longer disease-free survival, while the PD-L1-ICS of either ≥1% (HR 0.28; p = 0.04) or ≥5% (HR 0.19; p = 0.03) in surgical samples and the biopsy-to-surgery increase in PD-L1-ICS (HR: 0.20; p = 0.04) were associated with better overall survival. Our study suggests that PD-L1 expression in RC is largely reflective of immune cell infiltration, and its presence/increase in surgical samples predicts better outcomes.
Keywords: PD-L1; combined positive score (CPS); immune cell score (ICS); immune checkpoint inhibitors; radiotherapy; rectal cancer; tumour proportion score (TPS).
Conflict of interest statement
Alain Hendlisz: consultancy, advisory roles, and honoraria from Amgen, Bayer, Eli Lilly, Merck, Pierre Fabre, Servier, and Sirtex. Research funding (institutional) from Amgen, Astra Zeneca, Ipsen, Leo Pharma, Merck, Roche, Sanofi, and Teva Pharma. Support for travel/accommodation from Merck, Roche, and Sirtex. Francesco Sclafani: consultancy, advisory roles, and honoraria: AMAL Therapeutics, Amgen, Bayer, BMS, Dragonfly Therapeutics, GSK, Merck, Nordic Pharma, Roche, and Servier; research funding (institute): Amgen, Astellas, Astra Zeneca, Bayer, BMS, Merck, MSD, Pierre-Fabre, and Roche, Sanofi; travel grants: Amgen, Bayer, Lilly, Merck, Roche, and Servier; and leadership roles: Secretary EORTC Gastrointestinal Tract Cancer Group. All the other authors do not have any conflicts of interest.
Figures
References
-
- Dijkstra E.A., Nilsson P.J., Hospers G.A.P., Bahadoer R.R., Meershoek-Klein Kranenbarg E., Roodvoets A.G.H., Putter H., Berglund Å., Cervantes A., Crolla R.M.P.H., et al. Locoregional Failure during and after Short-course Radiotherapy followed by Chemotherapy and Surgery Compared with Long-course Chemoradiotherapy and Surgery: A 5-Year Follow-up of the RAPIDO Trial. Ann. Surg. 2023;278:e766–e772. doi: 10.1097/SLA.0000000000005799. - DOI - PMC - PubMed
-
- Conroy T., Etienne P.-L., Rio E., Evesque L., Mesgouez-Nebout N., Vendrely V., Artignan X., Bouche O., Boileve A., Delaye M., et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: 7-year results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J. Clin. Oncol. 2023;41((Suppl. S17)):LBA3504. doi: 10.1200/JCO.2023.41.17_suppl.LBA3504. - DOI
-
- Verheij F.S., Omer D.M.R., Williams H., Buckley J.T., Lin S.T., Qin L.-X., Thompson H.M., Yuval J.B., Gollub M.J., Wu A.J.-C., et al. Sustained organ preservation in patients with rectal cancer treated with total neoadjuvant therapy: Long-term results of the OPRA trial. J. Clin. Oncol. 2023;41((Suppl. S16)):3520. doi: 10.1200/JCO.2023.41.16_suppl.3520. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

