Exploring the Role of GITR/GITRL Signaling: From Liver Disease to Hepatocellular Carcinoma
- PMID: 39061246
- PMCID: PMC11275207
- DOI: 10.3390/cancers16142609
Exploring the Role of GITR/GITRL Signaling: From Liver Disease to Hepatocellular Carcinoma
Abstract
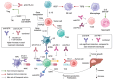
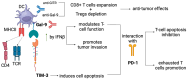
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and presents a continuously growing incidence and high mortality rates worldwide. Besides advances in diagnosis and promising results of pre-clinical studies, established curative therapeutic options for HCC are not currently available. Recent progress in understanding the tumor microenvironment (TME) interactions has turned the scientific interest to immunotherapy, revolutionizing the treatment of patients with advanced HCC. However, the limited number of HCC patients who benefit from current immunotherapeutic options creates the need to explore novel targets associated with improved patient response rates and potentially establish them as a part of novel combinatorial treatment options. Glucocorticoid-induced TNFR-related protein (GITR) belongs to the TNFR superfamily (TNFRSF) and promotes CD8+ and CD4+ effector T-cell function with simultaneous inhibition of Tregs function, when activated by its ligand, GITRL. GITR is currently considered a potential immunotherapy target in various kinds of neoplasms, especially with the concomitant use of programmed cell-death protein-1 (PD-1) blockade. Regarding liver disease, a high GITR expression in liver progenitor cells has been observed, associated with impaired hepatocyte differentiation, and decreased progenitor cell-mediated liver regeneration. Considering real-world data proving its anti-tumor effect and recently published evidence in pre-clinical models proving its involvement in pre-cancerous liver disease, the idea of its inclusion in HCC therapeutic options theoretically arises. In this review, we aim to summarize the current evidence supporting targeting GITR/GITRL signaling as a potential treatment strategy for advanced HCC.
Keywords: anti-GITR monoclonal antibodies; cancer; glucocorticoid-induced TNFR-related protein (GITR); hepatocellular carcinoma (HCC); immunotherapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Akinyemiju T., Abera S., Ahmed M., Alam N., Alemayohu M.A., Allen C., Al-Raddadi R., Alvis-Guzman N., Amoako Y., Artaman A. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

