New Fe3O4-Based Coatings with Enhanced Anti-Biofilm Activity for Medical Devices
- PMID: 39061313
- PMCID: PMC11273941
- DOI: 10.3390/antibiotics13070631
New Fe3O4-Based Coatings with Enhanced Anti-Biofilm Activity for Medical Devices
Abstract
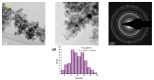
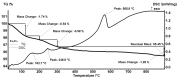
With the increasing use of invasive, interventional, indwelling, and implanted medical devices, healthcare-associated infections caused by pathogenic biofilms have become a major cause of morbidity and mortality. Herein, we present the fabrication, characterization, and in vitro evaluation of biocompatibility and anti-biofilm properties of new coatings based on Fe3O4 nanoparticles (NPs) loaded with usnic acid (UA) and ceftriaxone (CEF). Sodium lauryl sulfate (SLS) was employed as a stabilizer and modulator of the polarity, dispersibility, shape, and anti-biofilm properties of the magnetite nanoparticles. The resulting Fe3O4 functionalized NPs, namely Fe3O4@SLS, Fe3O4@SLS/UA, and Fe3O4@SLS/CEF, respectively, were prepared by co-precipitation method and fully characterized by XRD, TEM, SAED, SEM, FTIR, and TGA. They were further used to produce nanostructured coatings by matrix-assisted pulsed laser evaporation (MAPLE) technique. The biocompatibility of the coatings was assessed by measuring the cell viability, lactate dehydrogenase release, and nitric oxide level in the culture medium and by evaluating the actin cytoskeleton morphology of murine pre-osteoblasts. All prepared nanostructured coatings exhibited good biocompatibility. Biofilm growth inhibition ability was tested at 24 h and 48 h against Staphylococcus aureus and Pseudomonas aeruginosa as representative models for Gram-positive and Gram-negative bacteria. The coatings demonstrated good biocompatibility, promoting osteoblast adhesion, migration, and growth without significant impact on cell viability or morphology, highlighting their potential for developing safe and effective antibacterial surfaces.
Keywords: biofilm inhibition; ceftriaxone; coatings; hydrophobic nanoparticles; nosocomial infections; sodium lauryl sulfate; usnic acid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures













References
-
- Suetens A., Latour K., Kärki T., Ricchizzi E., Kinross P., Moro M.L., Jans B., Hopkins S., Hansen S., Lyytikäinen O., et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016 to 2017. Eurosurveillance. 2018;23:1800516. doi: 10.2807/1560-7917.ES.2018.23.46.1800516. - DOI - PMC - PubMed
-
- Cai S., Wu C., Yang W., Liang W., Yu H., Liu L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020;9:971–989. doi: 10.1515/ntrev-2020-0076. - DOI
LinkOut - more resources
Full Text Sources

