Verbal Learning and Memory Deficits across Neurological and Neuropsychiatric Disorders: Insights from an ENIGMA Mega Analysis
- PMID: 39061410
- PMCID: PMC11274572
- DOI: 10.3390/brainsci14070669
Verbal Learning and Memory Deficits across Neurological and Neuropsychiatric Disorders: Insights from an ENIGMA Mega Analysis
Abstract
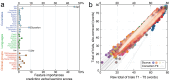
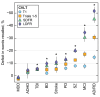
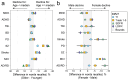
Deficits in memory performance have been linked to a wide range of neurological and neuropsychiatric conditions. While many studies have assessed the memory impacts of individual conditions, this study considers a broader perspective by evaluating how memory recall is differentially associated with nine common neuropsychiatric conditions using data drawn from 55 international studies, aggregating 15,883 unique participants aged 15-90. The effects of dementia, mild cognitive impairment, Parkinson's disease, traumatic brain injury, stroke, depression, attention-deficit/hyperactivity disorder (ADHD), schizophrenia, and bipolar disorder on immediate, short-, and long-delay verbal learning and memory (VLM) scores were estimated relative to matched healthy individuals. Random forest models identified age, years of education, and site as important VLM covariates. A Bayesian harmonization approach was used to isolate and remove site effects. Regression estimated the adjusted association of each clinical group with VLM scores. Memory deficits were strongly associated with dementia and schizophrenia (p < 0.001), while neither depression nor ADHD showed consistent associations with VLM scores (p > 0.05). Differences associated with clinical conditions were larger for longer delayed recall duration items. By comparing VLM across clinical conditions, this study provides a foundation for enhanced diagnostic precision and offers new insights into disease management of comorbid disorders.
Keywords: Parkinson’s disease; attention-deficit/hyperactivity disorder; bipolar disorder; dementia; depression; memory; schizophrenia; stroke; traumatic brain injury; verbal learning.
Conflict of interest statement
Celso Arango has been a consultant to or has received honoraria or grants from Abbot, Acadia, Ambrosetti, Angelini, Biogen, Boehringer, Gedeon Richter, Janssen Cilag, Lundbeck, Medscape, Menarini, Minerva, Otsuka, Pfizer, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion, Takeda, and Teva. Amy Brodtmann serves on the editorial boards of Neurology and International Journal of Stroke. Benedicto Crespo-Facorro has been a consultant to or has received honoraria or grants from Angelini, Boehringer, Johnson, Lundbeck, Otsuka, and Rovi. Covadonga M. Diaz-Caneja has received honoraria from Angelini and Viatris. Christopher C. Giza has been a consultant to the NBA, NFL, NHLPA, and Los Angeles Lakers, he has served on the advisory board for Highmark Interactive, Novartis, MLS, NBA, and USSF, and he handles 1–2 medicolegal cases annually. Jonathan Repple has received speaking honoraria from Janssen and Hexal. Jair C. Soares has been a consultant to, has received honoraria or grants from, or has stock in Alkermes, Allergan, Asofarma, ATAI, Boehringer Ingelheim, Compass, Johnson & Johnson, Livanova, Pfizer, Pulvinar Neuro LLC, Relmada, Sanofi, and Sunovian. Paul M. Thompson received partial research support from Biogen, Inc., for research unrelated to this manuscript. Lucy Vivash received partial research support for this project by an investigator-initiated research grant from Biogen (US). Biogen had no role in the analysis or writing of this manuscript. Additionally, Lucy Vivash received support from Eisai (JP) and Life Molecular Imaging for research unrelated to this manuscript. Glenn R. Wylie has received research support from the NJ Commission for Brain Injury Research, the Dept of Veterans Affairs, Biogen, Bristol, Myers, Squibb, and Genetech, and he has served on advisory boards for the CDMRP and the VA; all of these activities are unrelated to this research. Lakshmi N. Yatham has been on speaker or advisory boards for, or has received research grants from, Alkermes, Abbvie, Canadian Institutes of Health Research, Sumitomo Dainippon Pharma, GlaxoSmithKline, Intracellular Therapies, Merck, Sanofi, Sequiris, Servier, and Sunovion, all outside this work. David F. Tate received funding from the Defense and Veterans Brain Injury Centers, the U.S. Army Medical Research and Materiel Command, and the Chronic Effects of Neurotrauma Consortium (CENC). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. All other authors declare no conflicts of interest. The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
Figures