Evaluating the Effectiveness, Tolerability, and Safety of Eptinezumab in High-Frequency and Chronic Migraine in Real World: EMBRACE-The First Italian Multicenter, Prospective, Real-Life Study
- PMID: 39061413
- PMCID: PMC11275165
- DOI: 10.3390/brainsci14070672
Evaluating the Effectiveness, Tolerability, and Safety of Eptinezumab in High-Frequency and Chronic Migraine in Real World: EMBRACE-The First Italian Multicenter, Prospective, Real-Life Study
Abstract
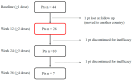
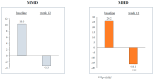
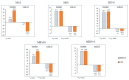
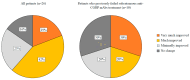
We conducted a multicenter, prospective study (EMBRACE) evaluating the real-life effectiveness, safety, and tolerability of eptinezumab (100 mg/300 mg)-a monoclonal antibody targeting the calcitonin-gene-related peptide (anti-CGRP mAb)-in high-frequency episodic migraine (HFEM) or chronic migraine (CM). The primary endpoint was the change in monthly migraine days (MMD) for HFEM or monthly headache days (MHD) for CM at weeks 9-12 compared to baseline. The secondary endpoints included changes in monthly analgesic intake (MAI), Numerical Rating Scale (NRS), Headache Impact Test (HIT-6), Migraine Disability Assessment Scale (MIDAS), Migraine Interictal Burden Scale (MIBS-4), and responder rates. The safety analysis involved 44 subjects; the effectiveness analysis included 26 individuals. Eptinezumab was well-tolerated. In CM patients, eptinezumab significantly reduced MHD (-16.1 ± 9.9, p < 0.001), MAI, NRS, HIT-6, MIDAS, and MIBS-4. In HFEM patients, it significantly reduced NRS, HIT-6, MIDAS, and MIBS-4, though reductions in MMD (-3.3 ± 4.5) and MAI were not statistically significant. Overall, ≥50% and ≥75% response rates were 61.5% and 30.8%, respectively (60% and 30% in non-responders to subcutaneous anti-CGRP mAbs). The clinical change was rated as much or very much improved by 61.0% of the patients. Eptinezumab demonstrated high effectiveness, safety, and tolerability in real-life among hard-to-treat migraine patients with multiple treatment failures, including anti-CGRP mAbs.
Keywords: anti-CGRP mAbs; disability; eptinezumab; migraine; real-life; treatment.
Conflict of interest statement
Piero Barbanti reports personal compensation for consulting, serving on a scientific advisory board, speaker panels, travel grants, research support, collaborated for clinical trials, or other activities with Abbvie, Alder, Allergan, Amgen, Angelini, Assosalute, Bayer, Biohaven, ElectroCore, Eli-Lilly, Fondazione Ricerca e Salute, GSK, Lundbeck, Lusofarmaco, 1MED, MSD, New Penta, Noema Pharma, Novartis, Pfizer, Stx-Med, Teva, Visufarma, and Zambon and serves as President of the Italian Association of Headache Sufferers. Bianca Orlando has no disclosures to declare. Gabriella Egeo received travel grants and honoraria from Eli-Lilly, Novartis, New Penta, and Ecupharma; Florindo d’Onofrio received travel grants, honoraria as a speaker or for participating in advisory boards from Novartis, Teva, Neopharmed Gentili, Qbgroup srl, K link srl, and Eli-Lilly; Alberto Doretti received travel grants and honoraria from Eli Lilly, Zambon, Teva, Abbvie, Neopharmed Gentili, and Lundbeck. Stefano Messina has no disclosures to declare. Massimo Autunno has no disclosures to declare. Roberta Messina received honoraria for speaker activities and participating in advisory boards from Abbvie, Biomedia, Eli Lilly, Lundbeck, Pfizer, and Teva. Massimo Filippi is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Neurological Sciences, and Radiology and received compensation for consulting services from Alexion, Almirall, Biogen, Merck, Novartis, Roche, Sanofi; speaking activities from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and TEVA; participation in Advisory Boards for Alexion, Biogen, Bristol Myers Squibb, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, and Takeda; scientific direction of educational events for Biogen, Merck, Roche, Celgene, Bristol-Myers Squibb, Lilly, Novartis, and Sanofi-Genzyme. He receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, the Italian Ministry of Health, the Italian Ministry of University and Research, and Fondazione Italiana Sclerosi Multipla. Giulia Fiorentini has no disclosures to declare. Cristina Rotondi has no disclosures to declare. Stefano Bonassi has no disclosures to declare. Cinzia Aurilia received travel grants from Eli-Lilly, FB-Health, Lusofarmaco, and Teva and honoraria from Novartis, Eli-Lilly, and Teva.
Figures

References
-
- Baker B., Schaeffler B., Beliveau M., Rubets I., Pederson S., Trinh M., Smith J., Latham J. Population pharmacokinetic and exposure-response analysis of eptinezumab in the treatment of episodic and chronic migraine. Pharmacol. Res. Perspect. 2020;8:e00567. doi: 10.1002/prp2.567. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

