Androgen-Induced, β-Catenin-Activated Hepatocellular Adenomatosis with Spontaneous External Rupture
- PMID: 39061609
- PMCID: PMC11276095
- DOI: 10.3390/diagnostics14141473
Androgen-Induced, β-Catenin-Activated Hepatocellular Adenomatosis with Spontaneous External Rupture
Abstract
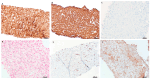
Androgens have long been recognized as oncogenic agents. They can induce both benign and malignant hepatocellular neoplasms, including hepatocellular adenoma (HCA) and hepatocellular carcinoma, though the underlying mechanisms remain unclear. Androgen-induced liver tumors are most often solitary and clinically silent. Herein, we reported an androgen-induced HCA complicated by spontaneous rupture. The patient was a 24-year-old male presenting with fatigue, diminished libido, radiology-diagnosed hepatocellular adenomatosis for 3 years, and sudden-onset, severe, sharp, constant abdominal pain for one day. He used Aveed (testosterone undecanoate injection) from age 17 and completely stopped one year before his presentation. A physical exam showed touch pain and voluntary guarding in the right upper quadrant of the abdomen. An abdominal CT angiogram demonstrated multiple probable HCAs, with active hemorrhage of the largest one (6.6 × 6.2 × 5.1 cm) accompanied by large-volume hemoperitoneum. After being stabilized by a massive transfusion protocol and interventional embolization, he underwent a percutaneous liver core biopsy. The biopsy specimen displayed atypical hepatocytes forming dense cords and pseudoglands. The lesional cells diffusely stained β-catenin in nuclei and glutamine synthetase in cytoplasm. Compared to normal hepatocytes from control tissue, the tumor cells were positive for nuclear AR (androgen receptor) expression but had no increased EZH2 (Enhancer of Zeste 2 Polycomb Repressive Complex 2 Subunit) protein expression. The case indicated that androgen-induced hepatocellular neoplasms should be included in the differential diagnosis of acute abdomen.
Keywords: EZH2; androgen; hepatocellualr adenomatosis; liver rupture; β-catenin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Nagtegaal I.D., Odze R.D., Klimstra D., Paradis V., Rugge M., Schirmacher P., Washington K.M., Carneiro F., Cree I.A., The WHO Classification of Tumours Editorial Board The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

