Evaluation and Limitations of the Novel Chemiluminescent Enzyme Immunoassay Technique for Measuring Total Tau Protein in the Cerebrospinal Fluid of Patients with Human Prion Disease: A 10-Year Prospective Study (2011-2020)
- PMID: 39061657
- PMCID: PMC11275853
- DOI: 10.3390/diagnostics14141520
Evaluation and Limitations of the Novel Chemiluminescent Enzyme Immunoassay Technique for Measuring Total Tau Protein in the Cerebrospinal Fluid of Patients with Human Prion Disease: A 10-Year Prospective Study (2011-2020)
Abstract
Background: Recently, the investigation of cerebrospinal fluid (CSF) biomarkers for diagnosing human prion diseases (HPD) has garnered significant attention. Reproducibility and accuracy are paramount in biomarker research, particularly in the measurement of total tau (T-tau) protein, which is a crucial diagnostic marker. Given the global impact of the coronavirus disease pandemic, the frequency of measuring this protein using one of the world's fully automated assays, chemiluminescent enzyme immunoassay (CLEA), has increased. At present, the diagnosis and monitoring of neurological diseases mainly rely on traditional methods, but their accuracy and responsiveness are limited. There is limited knowledge of the accuracy of CLEA in tau measurements. We aimed to measure T-tau protein using CLEA and to elucidate its merits and limitations.
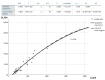
Methods: We randomly selected 60 patients with rapidly progressive dementia, using ELISA and CLEA analysis of cerebrospinal fluid specimens. Additionally, we used Western blotting to detect the presence of 14-3-3 protein and employed real-time quaking-induced conversion (RT-QuIC) assays to analyze the same set of samples. Furthermore, we examined the correlation coefficient between ELISA and CLEA results in a subset of 60 samples. Moreover, using CLEA, we evaluated the diurnal reproducibility, storage stability, dilutability, and freeze-thaw effects in three selected samples.
Results: In 172 patients, 172 samples were extracted, with each patient providing only one sample, and a total of 88 (35 men and 53 women) tested positive for HPD in the RT-QuIC assay. In contrast, all CSF samples from the remaining 84 patients without HPD (50 men and 34 women) tested negative in the RT-QuIC assay. Both ELISA and CLEA showed perfect sensitivity and specificity (100%) in measuring T-tau protein levels. In addition, ELISA and CLEA are similar in terms of measurement sensitivity and marginal effect of detection extrema. CLEA analysis exhibited instability for certain samples with T-tau protein levels exceeding 2000 pg/mL, leading to low reproducibility during dilution analysis.
Conclusions: Our findings indicate that CLEA outperforms ELISA in terms of diurnal reproducibility, storage stability, and freeze-thaw effects. However, ELISA demonstrated superior performance in the dilution assay. Therefore, it is imperative to develop innovative approaches for the dilution of biomarker samples for CLEA measurements during clinical trials.
Keywords: Creutzfeldt–Jakob disease; cerebrospinal fluid; chemiluminescent enzyme immunoassay; diagnostic test; human prion disease; prion protein.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures

References
-
- McGuire L.I., Poleggi A., Poggiolini I., Suardi S., Grznarova K., Shi S., Satoh K., Cheng K., Cramm M., Fairfoul G., et al. Cerebrospinal fluid real-time quaking-induced conversion is a robust and reliable test for sporadic Creutzfeldt–Jakob disease: An international study. Ann Neurol. 2016;80:160–165. doi: 10.1002/ana.24679. - DOI - PMC - PubMed
-
- Satoh K., Atarashi R., Nishida N. Real-time quaking-induced conversion for diagnosis of prion disease. Methods Mol. Biol. 2017;1658:305–310. - PubMed
Grants and funding
- 18ek0109362h0001 and JP20ak0101151/Research on Policy Planning and Evaluation for Rare and Intractable Diseases; Health and Labour Sciences Research Grants; the Research Committee of Surveillance and Infection Control of Prion Disease; and the Japan Agency for Medical Research and Developm
- KSat No. 14507303/research from the Ministry of Health, Labour, and Welfare of Japan
LinkOut - more resources
Full Text Sources

