Synergistic Cellular Responses Conferred by Concurrent Optical and Magnetic Stimulation Are Attenuated by Simultaneous Exposure to Streptomycin: An Antibiotic Dilemma
- PMID: 39061719
- PMCID: PMC11274164
- DOI: 10.3390/bioengineering11070637
Synergistic Cellular Responses Conferred by Concurrent Optical and Magnetic Stimulation Are Attenuated by Simultaneous Exposure to Streptomycin: An Antibiotic Dilemma
Abstract
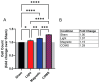
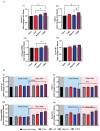
Concurrent optical and magnetic stimulation (COMS) combines extremely low-frequency electromagnetic and light exposure for enhanced wound healing. We investigated the potential mechanistic synergism between the magnetic and light components of COMS by comparing their individual and combined cellular responses. Lone magnetic field exposure produced greater enhancements in cell proliferation than light alone, yet the combined effects of magnetic fields and light were supra-additive of the individual responses. Reactive oxygen species were incrementally reduced by exposure to light, magnetics fields, and their combination, wherein statistical significance was only achieved by the combined COMS modality. By contrast, ATP production was most greatly enhanced by magnetic exposure in combination with light, indicating that mitochondrial respiratory efficiency was improved by the combination of magnetic fields plus light. Protein expression pertaining to cell proliferation was preferentially enhanced by the COMS modality, as were the protein levels of the TRPC1 cation channel that had been previously implicated as part of a calcium-mitochondrial signaling axis invoked by electromagnetic exposure and necessary for proliferation. These results indicate that light facilitates functional synergism with magnetic fields that ultimately impinge on mitochondria-dependent developmental responses. Aminoglycoside antibiotics (AGAs) have been previously shown to inhibit TRPC1-mediated magnetotransduction, whereas their influence over photomodulation has not been explored. Streptomycin applied during exposure to light, magnetic fields, or COMS reduced their respective proliferation enhancements, whereas streptomycin added after the exposure did not. Magnetic field exposure and the COMS modality were capable of partially overcoming the antagonism of proliferation produced by streptomycin treatment, whereas light alone was not. The antagonism of photon-electromagnetic effects by streptomycin implicates TRPC1-mediated calcium entry in both magnetotransduction and photomodulation. Avoiding the prophylactic use of AGAs during COMS therapy will be crucial for maintaining clinical efficacy and is a common concern in most other electromagnetic regenerative paradigms.
Keywords: aminoglycoside antibiotics; chronic wounds; hard-to-heal wounds; magnetic mitohormesis; magnetoreception; mitochondria; photomodulation; proliferation; reactive oxygen species; wound healing.
Conflict of interest statement
J.F. is a cofounder of Piomic Medical AG. J.F. is also the founder of Fields at Work GmbH, a company that specializes in designing and manufacturing magnetic radiation exposure and detection devices. Fields at Work GmbH operates independently of Piomic Medical AG. All other authors declare no conflicts of interest.
Figures


References
-
- Traber J., Wild T., Marotz J., Berli M.C., Franco-Obregon A. Concurrent Optical- and Magnetic-Stimulation-Induced Changes on Wound Healing Parameters, Analyzed by Hyperspectral Imaging: An Exploratory Case Series. Bioengineering. 2023;10:750. doi: 10.3390/bioengineering10070750. - DOI - PMC - PubMed
-
- Yap J.L.Y., Tai Y.K., Fröhlich J., Fong C.H.H., Yin J.N., Foo Z.L., Ramanan S., Beyer C., Toh S.J., Casarosa M., et al. Ambient and supplemental magnetic fields promote myogenesis via a TRPC1-mitochondrial axis: Evidence of a magnetic mitohormetic mechanism. FASEB J. 2019;33:12853–12872. doi: 10.1096/fj.201900057R. - DOI - PMC - PubMed
-
- Ma Q., Chen C., Deng P., Zhu G., Lin M., Zhang L., Xu S., He M., Lu Y., Duan W., et al. Extremely Low-Frequency Electromagnetic Fields Promote In Vitro Neuronal Differentiation and Neurite Outgrowth of Embryonic Neural Stem Cells via Up-Regulating TRPC1. PLoS ONE. 2016;11:e0150923. doi: 10.1371/journal.pone.0150923. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

