Radiomics and Deep Features: Robust Classification of Brain Hemorrhages and Reproducibility Analysis Using a 3D Autoencoder Neural Network
- PMID: 39061725
- PMCID: PMC11273742
- DOI: 10.3390/bioengineering11070643
Radiomics and Deep Features: Robust Classification of Brain Hemorrhages and Reproducibility Analysis Using a 3D Autoencoder Neural Network
Abstract
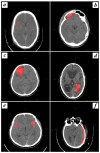
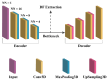
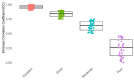
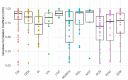
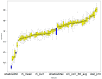
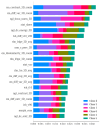
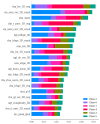
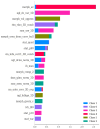
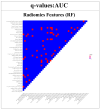
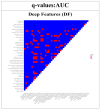
This study evaluates the reproducibility of machine learning models that integrate radiomics and deep features (features extracted from a 3D autoencoder neural network) to classify various brain hemorrhages effectively. Using a dataset of 720 patients, we extracted 215 radiomics features (RFs) and 15,680 deep features (DFs) from CT brain images. With rigorous screening based on Intraclass Correlation Coefficient thresholds (>0.75), we identified 135 RFs and 1054 DFs for analysis. Feature selection techniques such as Boruta, Recursive Feature Elimination (RFE), XGBoost, and ExtraTreesClassifier were utilized alongside 11 classifiers, including AdaBoost, CatBoost, Decision Trees, LightGBM, Logistic Regression, Naive Bayes, Neural Networks, Random Forest, Support Vector Machines (SVM), and k-Nearest Neighbors (k-NN). Evaluation metrics included Area Under the Curve (AUC), Accuracy (ACC), Sensitivity (SEN), and F1-score. The model evaluation involved hyperparameter optimization, a 70:30 train-test split, and bootstrapping, further validated with the Wilcoxon signed-rank test and q-values. Notably, DFs showed higher accuracy. In the case of RFs, the Boruta + SVM combination emerged as the optimal model for AUC, ACC, and SEN, while XGBoost + Random Forest excelled in F1-score. Specifically, RFs achieved AUC, ACC, SEN, and F1-scores of 0.89, 0.85, 0.82, and 0.80, respectively. Among DFs, the ExtraTreesClassifier + Naive Bayes combination demonstrated remarkable performance, attaining an AUC of 0.96, ACC of 0.93, SEN of 0.92, and an F1-score of 0.92. Distinguished models in the RF category included SVM with Boruta, Logistic Regression with XGBoost, SVM with ExtraTreesClassifier, CatBoost with XGBoost, and Random Forest with XGBoost, each yielding significant q-values of 42. In the DFs realm, ExtraTreesClassifier + Naive Bayes, ExtraTreesClassifier + Random Forest, and Boruta + k-NN exhibited robustness, with 43, 43, and 41 significant q-values, respectively. This investigation underscores the potential of synergizing DFs with machine learning models to serve as valuable screening tools, thereby enhancing the interpretation of head CT scans for patients with brain hemorrhages.
Keywords: brain; deep features; hemorrhage; machine learning; radiomics features; reproducible.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources

