Protective Effect of Ergothioneine against Oxidative Stress-Induced Chondrocyte Death
- PMID: 39061869
- PMCID: PMC11274255
- DOI: 10.3390/antiox13070800
Protective Effect of Ergothioneine against Oxidative Stress-Induced Chondrocyte Death
Abstract
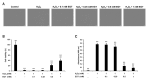
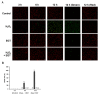
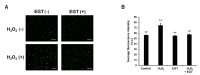
Reactive oxygen species (ROS) induce oxidative stress in cells and are associated with various diseases, including autoimmune diseases. Ergothioneine (EGT) is a natural amino acid derivative derived from the ergot fungus and has been reported to exhibit an effective antioxidant function in many models of oxidative stress-related diseases. Recently, mutations in OCTN1, a membrane transporter of EGT, have been reported to be associated with rheumatoid arthritis. Therefore, we investigated the chondrocyte-protective function of EGT using a model of oxidative stress-induced injury of chondrocytes by hydrogen peroxide (H2O2). Human chondrocytes were subjected to oxidative stress induced by H2O2 treatment, and cell viability, the activity of lactate dehydrogenase (LDH) released into the medium, dead cell ratio, intracellular ROS production, and mitochondrial morphology were assessed. EGT improved chondrocyte viability and LDH activity in the medium and strongly suppressed the dead cell ratio. EGT also exerted protective effects on intracellular ROS production and mitochondrial morphology. These results provide evidence to support the protective effects of EGT on chondrocytes induced by oxidative stress.
Keywords: chondrocyte protection; ergothioneine; oxidative stress; reactive oxygen species; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

