OxInflammatory Responses in the Wound Healing Process: A Systematic Review
- PMID: 39061892
- PMCID: PMC11274091
- DOI: 10.3390/antiox13070823
OxInflammatory Responses in the Wound Healing Process: A Systematic Review
Abstract
Significant sums are spent every year to find effective treatments to control inflammation and speed up the repair of damaged skin. This study investigated the main mechanisms involved in the skin wound cure. Consequently, it offered guidance to develop new therapies to control OxInflammation and infection and decrease functional loss and cost issues. This systematic review was conducted using the PRISMA guidelines, with a structured search in the MEDLINE (PubMed), Scopus, and Web of Science databases, analyzing 23 original studies. Bias analysis and study quality were assessed using the SYRCLE tool (Prospero number is CRD262 936). Our results highlight the activation of membrane receptors (IFN-δ, TNF-α, toll-like) in phagocytes, especially macrophages, during early wound healing. The STAT1, IP3, and NF-kβ pathways are positively regulated, while Ca2+ mobilization correlates with ROS production and NLRP3 inflammasome activation. This pathway activation leads to the proteolytic cleavage of caspase-1, releasing IL-1β and IL-18, which are responsible for immune modulation and vasodilation. Mediators such as IL-1, iNOS, TNF-α, and TGF-β are released, influencing pro- and anti-inflammatory cascades, increasing ROS levels, and inducing the oxidation of lipids, proteins, and DNA. During healing, the respiratory burst depletes antioxidant defenses (SOD, CAT, GST), creating a pro-oxidative environment. The IFN-δ pathway, ROS production, and inflammatory markers establish a positive feedback loop, recruiting more polymorphonuclear cells and reinforcing the positive interaction between oxidative stress and inflammation. This process is crucial because, in the immune system, the vicious positive cycle between ROS, the oxidative environment, and, above all, the activation of the NLRP3 inflammasome inappropriately triggers hypoxia, increases ROS levels, activates pro-inflammatory cytokines and inhibits the antioxidant action and resolution of anti-inflammatory cytokines, contributing to the evolution of chronic inflammation and tissue damage.
Keywords: inflammasome; inflammation; oxidative stress; wound closure.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
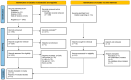
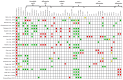

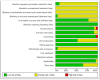
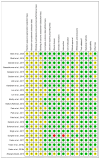


References
-
- Altoé L.S., Alves R.S., Miranda L.L., Sarandy M.M., Bastos D.S.S., Gonçalves-Santos E., Novaes R.D., Gonçalves R.V. Doxycycline Hyclate Modulates Antioxidant Defenses, Matrix Metalloproteinases, and COX-2 Activity Accelerating Skin Wound Healing by Secondary Intention in Rats. Oxidative Med. Cell. Longev. 2021;2021:4681041. doi: 10.1155/2021/4681041. - DOI - PMC - PubMed
-
- Alves R.S., Alves L.B., Altoé L.S., Sarandy M.M., Freitas M.B., Silveira N.J.F., Novaes R.D., Gonçalves R.V. Peptides from Animal Origin: A Systematic Review on Biological Sources and Effects on Skin Wounds. Oxidative Med. Cell. Longev. 2020;2020:4352761. doi: 10.1155/2020/4352761. - DOI - PMC - PubMed
-
- Laureano A., Rodrigues A.M. Cicatrização De Feridas. Revista da SPDV-Educação Médica Contínua. 2011;69:355. doi: 10.29021/spdv.69.3.71. - DOI
Publication types
Grants and funding
- APQ-03519-22, APQ-04164-22/Fundação de Amparo à Pesquisa do Estado de Minas Gerais
- 310413/2023-0/National Council for Scientific and Technological Development
- 403194/2023-7/National Council for Scientific and Technological Development
- 306733/2023-4/National Council for Scientific and Technological Development
- 001/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

