Citrus Pomace as a Source of Plant Complexes to Be Used in the Nutraceutical Field of Intestinal Inflammation
- PMID: 39061937
- PMCID: PMC11274116
- DOI: 10.3390/antiox13070869
Citrus Pomace as a Source of Plant Complexes to Be Used in the Nutraceutical Field of Intestinal Inflammation
Abstract
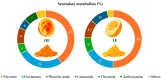
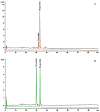
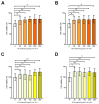
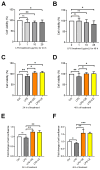
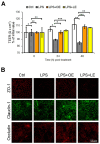
This study aims to recover the main by-product of Citrus fruits processing, the raw pomace, known also as pastazzo, to produce plant complexes to be used in the treatment of inflammatory bowel disease (IBD). Food-grade extracts from orange (OE) and lemon (LE) pomace were obtained by ultrasound-assisted maceration. After a preliminary phytochemical and biological screening by in vitro assays, primary and secondary metabolites were characterized by proton nuclear magnetic resonance (1H-NMR) and liquid chromatography coupled to diode array detection and electrospray ionization mass spectrometry (LC-DAD-ESI-MS) analyses. The intestinal bioaccessibility and antioxidant and anti-inflammatory properties were investigated by in vitro simulated gastro-intestinal digestion followed by treatments on a lipopolysaccharide (LPS)-stimulated human colorectal adenocarcinoma cell line (Caco-2). The tight junctions-associated structural proteins (ZO-1, Claudin-1, and Occludin), transepithelial electrical resistance (TEER), reactive oxygen species (ROS)-levels, expression of some key antioxidant (CAT, NRF2 and SOD2) and inflammatory (IL-1β, IL-6, TNF-α, IL-8) genes, and pNFkB p65 nuclear translocation, were evaluated. The OE and LE digesta, which did not show any significant difference in terms of phytochemical profile, showed significant effects in protecting against the LPS-induced intestinal barrier damage, oxidative stress and inflammatory response. In conclusion, both OE and LE emerged as potential candidates for further preclinical studies on in vivo IBD models.
Keywords: Citrus by-products; anti-inflammatory activity; antioxidant activity; food-grade extracts; in vitro simulated gastro-duodenal digestion; intestinal bioaccessibility; nutraceutics; phytochemistry; primary metabolites; secondary metabolites.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Maiuolo J., Bosco F., Guarnieri L., Nucera S., Ruga S., Oppedisano F., Tucci L., Muscoli C., Palma E., Giuffrè A.M., et al. Protective Role of an Extract Waste Product from Citrus bergamia in an In Vitro Model of Neurodegeneration. Plants. 2023;12:2126. doi: 10.3390/plants12112126. - DOI - PMC - PubMed
-
- Food and Agriculture Organization of the United Nations. [(accessed on 20 June 2024)]. Available online: https://www.fao.org/markets-and-trade/commodities/citrus/en/
LinkOut - more resources
Full Text Sources
Miscellaneous

