Suppressing Pro-Apoptotic Proteins by siRNA in Corneal Endothelial Cells Protects against Cell Death
- PMID: 39062012
- PMCID: PMC11274739
- DOI: 10.3390/biomedicines12071439
Suppressing Pro-Apoptotic Proteins by siRNA in Corneal Endothelial Cells Protects against Cell Death
Abstract
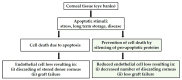
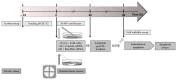
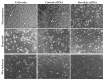
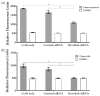
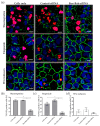
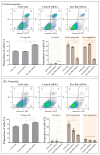
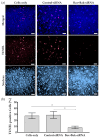
Corneal endothelial cells (CE) are critical for the cornea's transparency. For severe corneal damage, corneal tissue transplantation is the most promising option for restoring vision. However, CE apoptotic cell death occurs during the storage of donor corneas for transplantation. This study used small interfering (si)RNA-mediated silencing of pro-apoptotic proteins as a novel strategy to protect CE against apoptosis. Therefore, the pro-apoptotic proteins Bax and Bak were silenced in the human corneal endothelial cell line (HCEC-12) by transfection with Accell™siRNA without any adverse effects on cell viability. When apoptosis was induced, e.g., etoposide, the caspase-3 activity and Annexin V-FITC/PI assay indicated a significantly reduced apoptosis rate in Bax+Bak-siRNA transfected HCECs compared to control (w/o siRNA). TUNEL assay in HCECs exposed also significantly lower cell death in Bax+Bak-siRNA (7.5%) compared to control (w/o siRNA: 32.8%). In ex vivo donor corneas, a significant reduction of TUNEL-positive CEs in Bax+Bak-siRNA corneas (8.1%) was detectable compared to control-treated corneas (w/o siRNA: 27.9%). In this study, we demonstrated that suppressing pro-apoptotic siRNA leads to inhibiting CE apoptosis. Gene therapy with siRNA may open a new translational approach for corneal tissue treatment in the eye bank before transplantation, leading to graft protection and prolonged graft survival.
Keywords: Annexin V; TUNEL; anti-apoptotic Bax and Bak; apoptosis; caspase-3; confocal laser scanning microscopy; corneal endothelial cells; donor cornea; siRNA.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Heat shock protein 27 phosphorylation is involved in epithelial cell apoptosis as well as epithelial migration during corneal epithelial wound healing.Exp Eye Res. 2014 Jan;118:36-41. doi: 10.1016/j.exer.2013.11.002. Epub 2013 Nov 15. Exp Eye Res. 2014. PMID: 24239511
-
Silencing of Bak ameliorates apoptosis of human proximal tubular epithelial cells by Escherichia coli-derived Shiga toxin 2.Infection. 2005 Oct;33(5-6):362-7. doi: 10.1007/s15010-005-5073-5. Infection. 2005. PMID: 16258868
-
Apoptotic effects of norfloxacin on corneal endothelial cells.Naunyn Schmiedebergs Arch Pharmacol. 2020 Jan;393(1):77-88. doi: 10.1007/s00210-019-01711-5. Epub 2019 Aug 16. Naunyn Schmiedebergs Arch Pharmacol. 2020. PMID: 31420720
-
[Transplantation of corneal endothelial cells].Nippon Ganka Gakkai Zasshi. 2002 Dec;106(12):805-35; discussion 836. Nippon Ganka Gakkai Zasshi. 2002. PMID: 12610838 Review. Japanese.
-
Corneal endothelial regeneration and tissue engineering.Prog Retin Eye Res. 2013 Jul;35:1-17. doi: 10.1016/j.preteyeres.2013.01.003. Epub 2013 Jan 23. Prog Retin Eye Res. 2013. PMID: 23353595 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

