Cannabidiol and Beta-Caryophyllene Combination Attenuates Diabetic Neuropathy by Inhibiting NLRP3 Inflammasome/NFκB through the AMPK/sirT3/Nrf2 Axis
- PMID: 39062016
- PMCID: PMC11274582
- DOI: 10.3390/biomedicines12071442
Cannabidiol and Beta-Caryophyllene Combination Attenuates Diabetic Neuropathy by Inhibiting NLRP3 Inflammasome/NFκB through the AMPK/sirT3/Nrf2 Axis
Abstract
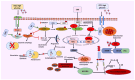
Background: In this study, we investigated in detail the role of cannabidiol (CBD), beta-caryophyllene (BC), or their combinations in diabetic peripheral neuropathy (DN). The key factors that contribute to DN include mitochondrial dysfunction, inflammation, and oxidative stress.
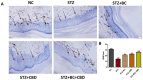
Methods: Briefly, streptozotocin (STZ) (55 mg/kg) was injected intraperitoneally to induce DN in Sprague-Dawley rats, and we performed procedures involving Randall Sellito calipers, a Von Frey aesthesiometer, a hot plate, and cold plate methods to determine mechanical and thermal hyperalgesia in vivo. The blood flow to the nerves was assessed using a laser Doppler device. Schwann cells were exposed to high glucose (HG) at a dose of 30 mM to induce hyperglycemia and DCFDA, and JC1 and Mitosox staining were performed to determine mitochondrial membrane potential, reactive oxygen species, and mitochondrial superoxides in vitro. The rats were administered BC (30 mg/kg), CBD (15 mg/kg), or combination via i.p. injections, while Schwann cells were treated with 3.65 µM CBD, 75 µM BC, or combination to assess their role in DN amelioration.
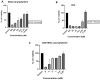
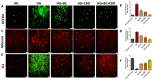
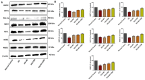
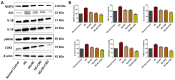
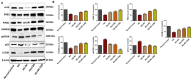
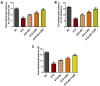
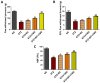
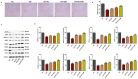
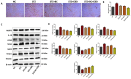
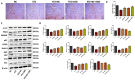
Results: Our results revealed that exposure to BC and CBD diminished HG-induced hyperglycemia in Schwann cells, in part by reducing mitochondrial membrane potential, reactive oxygen species, and mitochondrial superoxides. Furthermore, the BC and CBD combination treatment in vivo could prevent the deterioration of the mitochondrial quality control system by promoting autophagy and mitochondrial biogenesis while improving blood flow. CBD and BC treatments also reduced pain hypersensitivity to hyperalgesia and allodynia, with increased antioxidant and anti-inflammatory action in diabetic rats. These in vivo effects were attributed to significant upregulation of AMPK, sirT3, Nrf2, PINK1, PARKIN, LC3B, Beclin1, and TFAM functions, while downregulation of NLRP3 inflammasome, NFκB, COX2, and p62 activity was noted using Western blotting.
Conclusions: the present study demonstrated that STZ and HG-induced oxidative and nitrosative stress play a crucial role in the pathogenesis of diabetic neuropathy. We find, for the first time, that a CBD and BC combination ameliorates DN by modulating the mitochondrial quality control system.
Keywords: AMPK; NLRP3; autophagy; beta-caryophyllene; cannabidiol; mitochondrial biogenesis; sirT3.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Piceatannol promotes neuroprotection by inducing mitophagy and mitobiogenesis in the experimental diabetic peripheral neuropathy and hyperglycemia-induced neurotoxicity.Int Immunopharmacol. 2023 Mar;116:109793. doi: 10.1016/j.intimp.2023.109793. Epub 2023 Feb 1. Int Immunopharmacol. 2023. PMID: 36731149
-
Adenosine Monophosphate-Activated Protein Kinase Abates Hyperglycaemia-Induced Neuronal Injury in Experimental Models of Diabetic Neuropathy: Effects on Mitochondrial Biogenesis, Autophagy and Neuroinflammation.Mol Neurobiol. 2017 Apr;54(3):2301-2312. doi: 10.1007/s12035-016-9824-3. Epub 2016 Mar 8. Mol Neurobiol. 2017. PMID: 26957299
-
Salidroside alleviates diabetic neuropathic pain through regulation of the AMPK-NLRP3 inflammasome axis.Toxicol Appl Pharmacol. 2021 Apr 1;416:115468. doi: 10.1016/j.taap.2021.115468. Epub 2021 Feb 25. Toxicol Appl Pharmacol. 2021. PMID: 33639149
-
Adenosine monophosphate-activated protein kinase modulation by berberine attenuates mitochondrial deficits and redox imbalance in experimental diabetic neuropathy.Neuropharmacology. 2018 Mar 15;131:256-270. doi: 10.1016/j.neuropharm.2017.12.029. Epub 2017 Dec 20. Neuropharmacology. 2018. PMID: 29273519
-
Cannabidiol activates PINK1-Parkin-dependent mitophagy and mitochondrial-derived vesicles.Eur J Cell Biol. 2022 Jan;101(1):151185. doi: 10.1016/j.ejcb.2021.151185. Epub 2021 Nov 14. Eur J Cell Biol. 2022. PMID: 34915361 Free PMC article. Review.
Cited by
-
Identification of key genes and immune infiltration of diabetic peripheral neuropathy in mice and humans based on bioinformatics analysis.Front Endocrinol (Lausanne). 2024 Nov 18;15:1437979. doi: 10.3389/fendo.2024.1437979. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39624823 Free PMC article.
-
Diabetic peripheral neuropathy based on Schwann cell injury: mechanisms of cell death regulation and therapeutic perspectives.Front Endocrinol (Lausanne). 2024 Aug 12;15:1427679. doi: 10.3389/fendo.2024.1427679. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39193373 Free PMC article. Review.
-
Anti-Obesity Effects of LB-GABA.Int J Mol Sci. 2025 Apr 10;26(8):3554. doi: 10.3390/ijms26083554. Int J Mol Sci. 2025. PMID: 40332049 Free PMC article.
-
Intraplantar β-Caryophyllene Alleviates Pain and Inflammation in STZ-Induced Diabetic Peripheral Neuropathy via CB2 Receptor Activation.Int J Mol Sci. 2025 May 7;26(9):4430. doi: 10.3390/ijms26094430. Int J Mol Sci. 2025. PMID: 40362667 Free PMC article.
-
Cannabidiol Treatment in a Predator-Based Animal Model of PTSD: Assessing Oxidative Stress and Memory Performance.Int J Mol Sci. 2025 May 8;26(10):4491. doi: 10.3390/ijms26104491. Int J Mol Sci. 2025. PMID: 40429636 Free PMC article.
References
-
- Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B.B., Stein C., Basit A., Chan J.C.N., Mbanya J.C., et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

