Anti-Inflammatory and Cancer-Preventive Potential of Chamomile (Matricaria chamomilla L.): A Comprehensive In Silico and In Vitro Study
- PMID: 39062057
- PMCID: PMC11275008
- DOI: 10.3390/biomedicines12071484
Anti-Inflammatory and Cancer-Preventive Potential of Chamomile (Matricaria chamomilla L.): A Comprehensive In Silico and In Vitro Study
Erratum in
-
Correction: Drif et al. Anti-Inflammatory and Cancer-Preventive Potential of Chamomile (Matricaria chamomilla L.): A Comprehensive In Silico and In Vitro Study. Biomedicines 2024, 12, 1484.Biomedicines. 2025 Jun 30;13(7):1595. doi: 10.3390/biomedicines13071595. Biomedicines. 2025. PMID: 40679796 Free PMC article.
Abstract
Background and aim: Chamomile tea, renowned for its exquisite taste, has been appreciated for centuries not only for its flavor but also for its myriad health benefits. In this study, we investigated the preventive potential of chamomile (Matricaria chamomilla L.) towards cancer by focusing on its anti-inflammatory activity.
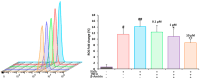
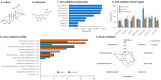
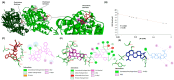
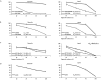
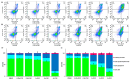
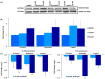
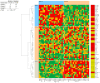
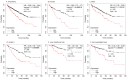
Methods and results: A virtual drug screening of 212 phytochemicals from chamomile revealed β-amyrin, β-eudesmol, β-sitosterol, apigenin, daucosterol, and myricetin as potent NF-κB inhibitors. The in silico results were verified through microscale thermophoresis, reporter cell line experiments, and flow cytometric determination of reactive oxygen species and mitochondrial membrane potential. An oncobiogram generated through comparison of 91 anticancer agents with known modes of action using the NCI tumor cell line panel revealed significant relationships of cytotoxic chamomile compounds, lupeol, and quercetin to microtubule inhibitors. This hypothesis was verified by confocal microscopy using α-tubulin-GFP-transfected U2OS cells and molecular docking of lupeol and quercetin to tubulins. Both compounds induced G2/M cell cycle arrest and necrosis rather than apoptosis. Interestingly, lupeol and quercetin were not involved in major mechanisms of resistance to established anticancer drugs (ABC transporters, TP53, or EGFR). Performing hierarchical cluster analyses of proteomic expression data of the NCI cell line panel identified two sets of 40 proteins determining sensitivity and resistance to lupeol and quercetin, further pointing to the multi-specific nature of chamomile compounds. Furthermore, lupeol, quercetin, and β-amyrin inhibited the mRNA expression of the proinflammatory cytokines IL-1β and IL6 in NF-κB reporter cells (HEK-Blue Null1). Moreover, Kaplan-Meier-based survival analyses with NF-κB as the target protein of these compounds were performed by mining the TCGA-based KM-Plotter repository with 7489 cancer patients. Renal clear cell carcinomas (grade 3, low mutational rate, low neoantigen load) were significantly associated with shorter survival of patients, indicating that these subgroups of tumors might benefit from NF-κB inhibition by chamomile compounds.
Conclusion: This study revealed the potential of chamomile, positioning it as a promising preventive agent against inflammation and cancer. Further research and clinical studies are recommended.
Keywords: Kaplan–Meier survival analysis; anti-inflammatory; carcinogenesis; cytokines; flavonoids; microscale thermophoresis; natural products; prevention; proteomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

