Osteopontin: A Key Multifaceted Regulator in Tumor Progression and Immunomodulation
- PMID: 39062100
- PMCID: PMC11274826
- DOI: 10.3390/biomedicines12071527
Osteopontin: A Key Multifaceted Regulator in Tumor Progression and Immunomodulation
Abstract
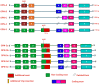
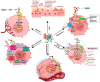
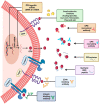
The tumor microenvironment (TME) is composed of various cellular components such as tumor cells, stromal cells including fibroblasts, adipocytes, mast cells, lymphatic vascular cells and infiltrating immune cells, macrophages, dendritic cells and lymphocytes. The intricate interplay between these cells influences tumor growth, metastasis and therapy failure. Significant advancements in breast cancer therapy have resulted in a substantial decrease in mortality. However, existing cancer treatments frequently result in toxicity and nonspecific side effects. Therefore, improving targeted drug delivery and increasing the efficacy of drugs is crucial for enhancing treatment outcome and reducing the burden of toxicity. In this review, we have provided an overview of how tumor and stroma-derived osteopontin (OPN) plays a key role in regulating the oncogenic potential of various cancers including breast. Next, we dissected the signaling network by which OPN regulates tumor progression through interaction with selective integrins and CD44 receptors. This review addresses the latest advancements in the roles of splice variants of OPN in cancer progression and OPN-mediated tumor-stromal interaction, EMT, CSC enhancement, immunomodulation, metastasis, chemoresistance and metabolic reprogramming, and further suggests that OPN might be a potential therapeutic target and prognostic biomarker for the evolving landscape of cancer management.
Keywords: cancer; cancer-associated fibroblasts; immunomodulation; osteopontin (OPN); single cell transcriptomics; targeted therapy; tumor-associated macrophages.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

