Simultaneous Expression of Different Therapeutic Genes by Infection with Multiple Oncolytic HSV-1 Vectors
- PMID: 39062150
- PMCID: PMC11274547
- DOI: 10.3390/biomedicines12071577
Simultaneous Expression of Different Therapeutic Genes by Infection with Multiple Oncolytic HSV-1 Vectors
Abstract
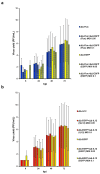
Oncolytic viruses (OVs) are anti-cancer therapeutics combining the selective killing of cancer cells with the triggering of an anti-tumoral immune response. The latter effect can be improved by arming OVs with immunomodulatory factors. Due to the heterogeneity of cancer and the tumor microenvironment, it is anticipated that strategies based on the co-expression of multiple therapeutic molecules that interfere with different features of the target malignancy will be more effective than mono-therapies. Here, we show that (i) the simultaneous expression of different proteins in triple-negative breast cancer (TNBC) cells can be achieved through their infection with a combination of OVs based on herpes simplex virus type 1 (oHSV1), each encoding a single transgene. (ii) The level of expressed proteins is dependent on the number of infectious viral particles utilized to challenge tumor cells. (iii) All recombinant viruses exhibited comparable efficacy in the killing of TNBC cells in single and multiple infections and showed similar kinetics of replication. Overall, our results suggest that a strategy based on co-infection with a panel of oHSV1s may represent a promising combinatorial therapeutic approach for TNBC, as well as for other types of solid tumors, that merits further investigation in more advanced in vitro and in vivo models.
Keywords: HSV-1; combinatorial approach; immunotherapy; virotherapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

