Exploring the Link between Varicella-Zoster Virus, Autoimmune Diseases, and the Role of Recombinant Zoster Vaccine
- PMID: 39062454
- PMCID: PMC11274381
- DOI: 10.3390/biom14070739
Exploring the Link between Varicella-Zoster Virus, Autoimmune Diseases, and the Role of Recombinant Zoster Vaccine
Abstract
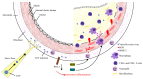
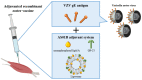
The varicella-zoster virus (VZV) is a human neurotropic herpes virus responsible for varicella and herpes zoster (HZ). Following primary infection in childhood, VZV manifests as varicella (chickenpox) and enters a period of latency within the dorsal root ganglion. A compromised cellular immune response due to aging or immunosuppression triggers viral reactivation and the development of HZ (shingles). Patients with autoimmune diseases have a higher risk of developing HZ owing to the immunodeficiency associated with the disease itself and/or the use of immunosuppressive agents. The introduction of new immunosuppressive agents with unique mechanisms has expanded the treatment options for autoimmune diseases but has also increased the risk of HZ. Specifically, Janus kinase (JAK) inhibitors and anifrolumab have raised concerns regarding HZ. Despite treatment advances, a substantial number of patients suffer from complications such as postherpetic neuralgia for prolonged periods. The adjuvanted recombinant zoster vaccine (RZV) is considered safe and effective even in immunocompromised patients. The widespread adoption of RZV may reduce the health and socioeconomic burdens of HZ patients. This review covers the link between VZV and autoimmune diseases, assesses the risk of HZ associated with immunosuppressant use, and discusses the benefits and risks of using RZV in patients with autoimmune diseases.
Keywords: Janus kinase inhibitors; autoimmune diseases; herpes zoster; recombinant zoster vaccine.
Conflict of interest statement
R.W. received a research grant from AbbVie and speaker’s fee from AbbVie, Asahi Kasei, Chugai, Eli Lilly, GSK, and UCB Japan. T.O. received a research grant and/or speaker’s fee from AbbVie, Asahi Kasei, Chugai, Eisai, Eli Lilly, Janssen, Novartis Pharma, and Tanabe Mitsubishi. M.H. received research grants and/or speaker fees from AbbVie, Asahi Kasei, Astellas, Brystol Meyers, Chugai, EA Pharma, Eisai, Daiichi Sankyo, Eli Lilly, Novartis Pharma, Taisho Toyama, and Tanabe Mitsubishi. Other authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Papaevangelou V., Quinlivan M., Lockwood J., Papaloukas O., Sideri G., Critselis E., Papassotiriou I., Papadatos J., Breuer J. Subclinical VZV reactivation in immunocompetent children hospitalized in the ICU associated with prolonged fever duration. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2013;19:E245–E251. doi: 10.1111/1469-0691.12131. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

