Molecular Mechanisms and Potential Antiviral Strategies of Liquid-Liquid Phase Separation during Coronavirus Infection
- PMID: 39062463
- PMCID: PMC11274562
- DOI: 10.3390/biom14070748
Molecular Mechanisms and Potential Antiviral Strategies of Liquid-Liquid Phase Separation during Coronavirus Infection
Abstract
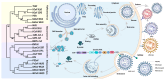
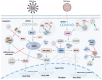
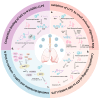
Highly pathogenic coronaviruses have caused significant outbreaks in humans and animals, posing a serious threat to public health. The rapid global spread of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has resulted in millions of infections and deaths. However, the mechanisms through which coronaviruses evade a host's antiviral immune system are not well understood. Liquid-liquid phase separation (LLPS) is a recently discovered mechanism that can selectively isolate cellular components to regulate biological processes, including host antiviral innate immune signal transduction pathways. This review focuses on the mechanism of coronavirus-induced LLPS and strategies for utilizing LLPS to evade the host antiviral innate immune response, along with potential antiviral therapeutic drugs and methods. It aims to provide a more comprehensive understanding and novel insights for researchers studying LLPS induced by pandemic viruses.
Keywords: SARS-CoV-2; innate immunity; liquid–liquid phase separation; nucleocapsid protein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses.Drug Resist Updat. 2020 Dec;53:100721. doi: 10.1016/j.drup.2020.100721. Epub 2020 Aug 26. Drug Resist Updat. 2020. PMID: 33132205 Free PMC article. Review.
-
Regulation of phase separation and antiviral activity of Cactin by glycolytic enzyme PGK via phosphorylation in Drosophila.mBio. 2024 Apr 10;15(4):e0137823. doi: 10.1128/mbio.01378-23. Epub 2024 Mar 6. mBio. 2024. PMID: 38446061 Free PMC article.
-
Induction of the Antiviral Immune Response and Its Circumvention by Coronaviruses.Viruses. 2020 Sep 18;12(9):1039. doi: 10.3390/v12091039. Viruses. 2020. PMID: 32961897 Free PMC article. Review.
-
Toll-Like Receptor 3 Signaling via TRIF Contributes to a Protective Innate Immune Response to Severe Acute Respiratory Syndrome Coronavirus Infection.mBio. 2015 May 26;6(3):e00638-15. doi: 10.1128/mBio.00638-15. mBio. 2015. PMID: 26015500 Free PMC article.
-
Insight into the mechanisms of coronaviruses evading host innate immunity.Biochim Biophys Acta Mol Basis Dis. 2023 Jun;1869(5):166671. doi: 10.1016/j.bbadis.2023.166671. Epub 2023 Feb 27. Biochim Biophys Acta Mol Basis Dis. 2023. PMID: 36858323 Free PMC article. Review.
Cited by
-
SARS-CoV-2 point mutations are over-represented in terminal loops of RNA stem-loop structures that can be resolved by Nsp13 helicase in a unique manner with respect to nucleotide dependence.Nucleic Acids Res. 2025 May 22;53(10):gkaf447. doi: 10.1093/nar/gkaf447. Nucleic Acids Res. 2025. PMID: 40421800 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

