Helicobacter pylori-Induced Decrease in Membrane Expression of Na,K-ATPase Leads to Gastric Injury
- PMID: 39062486
- PMCID: PMC11274427
- DOI: 10.3390/biom14070772
Helicobacter pylori-Induced Decrease in Membrane Expression of Na,K-ATPase Leads to Gastric Injury
Abstract
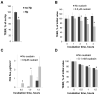
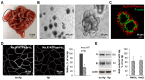
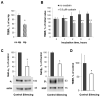
Helicobacter pylori is a highly prevalent human gastric pathogen that causes gastritis, ulcer disease, and gastric cancer. It is not yet fully understood how H. pylori injures the gastric epithelium. The Na,K-ATPase, an essential transporter found in virtually all mammalian cells, has been shown to be important for maintaining the barrier function of lung and kidney epithelia. H. pylori decreases levels of Na,K-ATPase in the plasma membrane of gastric epithelial cells, and the aim of this study was to demonstrate that this reduction led to gastric injury by impairing the epithelial barrier. Similar to H. pylori infection, the inhibition of Na,K-ATPase with ouabain decreased transepithelial electrical resistance and increased paracellular permeability in cell monolayers of human gastric cultured cells, 2D human gastric organoids, and gastric epithelium isolated from gerbils. Similar effects were caused by a partial shRNA silencing of Na,K-ATPase in human gastric organoids. Both H. pylori infection and ouabain exposure disrupted organization of adherens junctions in human gastric epithelia as demonstrated by E-cadherin immunofluorescence. Functional and structural impairment of epithelial integrity with a decrease in Na,K-ATPase amount or activity provides evidence that the H. pylori-induced downregulation of Na,K-ATPase plays a role in the complex mechanism of gastric disease induced by the bacteria.
Keywords: Helicobacter pylori; Na,K-ATPase; adherens junctions; gastric injury; ouabain.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

