Zinc, Copper, and Calcium: A Triangle in the Synapse for the Pathogenesis of Vascular-Type Senile Dementia
- PMID: 39062487
- PMCID: PMC11274390
- DOI: 10.3390/biom14070773
Zinc, Copper, and Calcium: A Triangle in the Synapse for the Pathogenesis of Vascular-Type Senile Dementia
Abstract
Zinc (Zn) and copper (Cu) are essential for normal brain functions. In particular, Zn and Cu are released to synaptic clefts during neuronal excitation. Synaptic Zn and Cu regulate neuronal excitability, maintain calcium (Ca) homeostasis, and play central roles in memory formation. However, in pathological conditions such as transient global ischemia, excess Zn is secreted to synaptic clefts, which causes neuronal death and can eventually trigger the pathogenesis of a vascular type of senile dementia. We have previously investigated the characteristics of Zn-induced neurotoxicity and have demonstrated that low concentrations of Cu can exacerbate Zn neurotoxicity. Furthermore, during our pharmacological approaches to clarify the molecular pathways of Cu-enhanced Zn-induced neurotoxicity, we have revealed the involvement of Ca homeostasis disruption. In the present review, we discuss the roles of Zn and Cu in the synapse, as well as the crosstalk between Zn, Cu, and Ca, which our study along with other recent studies suggest may underlie the pathogenesis of vascular-type senile dementia.
Keywords: calcium homeostasis; endoplasmic reticulum stress; ischemia; reactive oxygen species (ROS).
Conflict of interest statement
All authors declare that there are no conflicts of interest.
Figures


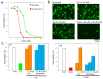

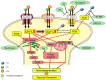
Similar articles
-
Crosstalk of copper and zinc in the pathogenesis of vascular dementia.J Clin Biochem Nutr. 2022 Jul;71(1):7-15. doi: 10.3164/jcbn.22-40. Epub 2022 Jul 1. J Clin Biochem Nutr. 2022. PMID: 35903609 Free PMC article.
-
Disruption of zinc homeostasis and the pathogenesis of senile dementia.Metallomics. 2014 Feb;6(2):209-19. doi: 10.1039/c3mt00257h. Metallomics. 2014. PMID: 24247360 Review.
-
Copper as a Collaborative Partner of Zinc-Induced Neurotoxicity in the Pathogenesis of Vascular Dementia.Int J Mol Sci. 2021 Jul 6;22(14):7242. doi: 10.3390/ijms22147242. Int J Mol Sci. 2021. PMID: 34298862 Free PMC article. Review.
-
The molecular mechanisms of zinc neurotoxicity and the pathogenesis of vascular type senile dementia.Int J Mol Sci. 2013 Nov 7;14(11):22067-81. doi: 10.3390/ijms141122067. Int J Mol Sci. 2013. PMID: 24213606 Free PMC article. Review.
-
Copper Enhances Zinc-Induced Neurotoxicity and the Endoplasmic Reticulum Stress Response in a Neuronal Model of Vascular Dementia.Front Neurosci. 2017 Feb 9;11:58. doi: 10.3389/fnins.2017.00058. eCollection 2017. Front Neurosci. 2017. PMID: 28232787 Free PMC article.
Cited by
-
Concentration-Based Analysis of Metal-Induced Tau Fibrillar versus non-fibrillar Aggregation: Implications for Neurotoxicity in Alzheimer's Disease.ChemistryOpen. 2025 Aug;14(8):e202400493. doi: 10.1002/open.202400493. Epub 2025 Mar 24. ChemistryOpen. 2025. PMID: 40125852 Free PMC article.
-
Elucidating molecular pathogenesis and developing targeted therapeutic interventions for cerebrovascular endothelial cell-mediated vascular dementia.Front Aging Neurosci. 2025 Jul 16;17:1623050. doi: 10.3389/fnagi.2025.1623050. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40741047 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

