Anti-Biofilm Perspectives of Propolis against Staphylococcus epidermidis Infections
- PMID: 39062493
- PMCID: PMC11274400
- DOI: 10.3390/biom14070779
Anti-Biofilm Perspectives of Propolis against Staphylococcus epidermidis Infections
Abstract
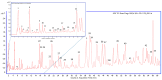
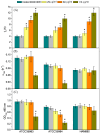
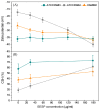
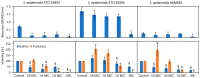
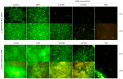
Staphylococcus epidermis has emerged as the main causative agent of medical device-related infections. Their major pathogenicity factor lies in its ability to adhere to surfaces and proliferate into biofilms, which increase their resistance to antibiotics. The main objective of this study was to evaluate the use and the mechanism of action of an ethanolic extract of Spanish propolis (EESP) as a potential alternative for preventing biofilm-related infections caused by S. epidermidis. The chemical composition of propolis is reported and its antibacterial activity against several strains of S. epidermidis with different biofilm-forming capacities evaluated. The influence of sub-inhibitory concentrations (sub-MICs) of EESP on their growth, physicochemical surface properties, adherence, and biofilm formation were studied. EESP interferes with planktonic cells, homogenizing their physicochemical surface properties and introducing a significant delay in their growth. The adherence and biofilms at the EESP concentrations investigated were decreased up to 90.5% among the strains. Microscopic analysis indicated that the planktonic cells that survived the treatment were the ones that adhere and proliferate on the surfaces. The results obtained suggest that the EESP has a high potential to be used as an inhibitor of both the adhesion and biofilm formation of S. epidermidis.
Keywords: Staphylococcus epidermidis; biofilms; hydrophobicity; propolis; zeta potential.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

