Alginate-Based Electrospun Nanofibers and the Enabled Drug Controlled Release Profiles: A Review
- PMID: 39062503
- PMCID: PMC11274620
- DOI: 10.3390/biom14070789
Alginate-Based Electrospun Nanofibers and the Enabled Drug Controlled Release Profiles: A Review
Abstract
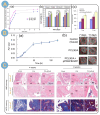
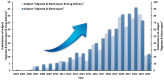
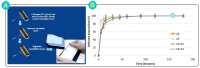
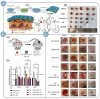
Alginate is a natural polymer with good biocompatible properties and is a potential polymeric material for the sustainable development and replacement of petroleum derivatives. However, the non-spinnability of pure alginate solutions has hindered the expansion of alginate applications. With the continuous development of electrospinning technology, synthetic polymers, such as PEO and PVA, are used as co-spinning agents to increase the spinnability of alginate. Moreover, the coaxial, parallel Janus, tertiary and other diverse and novel electrospun fiber structures prepared by multi-fluid electrospinning have found a new breakthrough for the problem of poor spinning of natural polymers. Meanwhile, the diverse electrospun fiber structures effectively achieve multiple release modes of drugs. The powerful combination of alginate and electrostatic spinning is widely used in many biomedical fields, such as tissue engineering, regenerative engineering, bioscaffolds, and drug delivery, and the research fever continues to climb. This is particularly true for the controlled delivery aspect of drugs. This review provides a brief overview of alginate, introduces new advances in electrostatic spinning, and highlights the research progress of alginate-based electrospun nanofibers in achieving various controlled release modes, such as pulsed release, sustained release, biphasic release, responsive release, and targeted release.
Keywords: alginate; biomedical; controlled release; drug delivery; electrospinning; nanostructure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Rezaei S., Valipouri A., Hosseini Ravandi S.A., Kouhi M., Ghasemi Mobarakeh L. Fabrication, Characterization, and Drug Release Study of Vitamin C–Loaded Alginate/Polyethylene Oxide Nanofibers for the Treatment of a Skin Disorder. Polym. Adv. Technol. 2019;30:2447–2457. doi: 10.1002/pat.4692. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

