Role of Type I Interferons during Mycobacterium tuberculosis and HIV Infections
- PMID: 39062562
- PMCID: PMC11275242
- DOI: 10.3390/biom14070848
Role of Type I Interferons during Mycobacterium tuberculosis and HIV Infections
Abstract
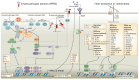
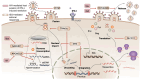
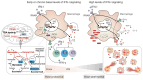
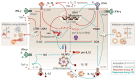
Tuberculosis and AIDS remain two of the most relevant human infectious diseases. The pathogens that cause them, Mycobacterium tuberculosis (Mtb) and HIV, individually elicit an immune response that treads the line between beneficial and detrimental to the host. Co-infection further complexifies this response since the different cytokines acting on one infection might facilitate the dissemination of the other. In these responses, the role of type I interferons is often associated with antiviral mechanisms, while for bacteria such as Mtb, their importance and clinical relevance as a suitable target for manipulation are more controversial. In this article, we review the recent knowledge on how these interferons play distinct roles and sometimes have opposite consequences depending on the stage of the pathogenesis. We highlight the dichotomy between the acute and chronic infections displayed by both infections and how type I interferons contribute to an initial control of each infection individually, while their chronic induction, particularly during HIV infection, might facilitate Mtb primo-infection and progression to disease. We expect that further findings and their systematization will allow the definition of windows of opportunity for interferon manipulation according to the stage of infection, contributing to pathogen clearance and control of immunopathology.
Keywords: HIV; co-infection; interferons; tuberculosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Nagano Y., Kojima Y., Sawai Y. Immunity and interference in vaccinia; inhibition of skin infection by inactivated virus. Comptes Rendus Seances Soc. Biol. Fil. 1954;148:750–752. - PubMed
-
- Biondo C., Midiri A., Gambuzza M., Gerace E., Falduto M., Galbo R., Bellantoni A., Beninati C., Teti G., Leanderson T., et al. IFN-α/β Signaling Is Required for Polarization of Cytokine Responses toward a Protective Type 1 Pattern during Experimental Cryptococcosis. J. Immunol. 2008;181:566–573. doi: 10.4049/jimmunol.181.1.566. - DOI - PubMed
-
- Ishihara T., Aga M., Hino K., Ushio C., Taniguchi M., Iwaki K., Ikeda M., Kurimoto M. Inhibition of Chlamydia trachomatis growth by human interferon-alpha;: Mechanisms and synergistic effect with interferon-gamma; and tumor necrosis factor-alpha. Biomed. Res. 2005;26:179–185. doi: 10.2220/biomedres.26.179. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

