De Novo Pathogenic Variant in FBRSL1, Non OMIM Gene Paralogue AUTS2, Causes a Novel Recognizable Syndromic Manifestation with Intellectual Disability; An Additional Patient and Review of the Literature
- PMID: 39062605
- PMCID: PMC11275389
- DOI: 10.3390/genes15070826
De Novo Pathogenic Variant in FBRSL1, Non OMIM Gene Paralogue AUTS2, Causes a Novel Recognizable Syndromic Manifestation with Intellectual Disability; An Additional Patient and Review of the Literature
Abstract
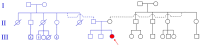
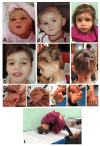
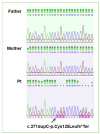
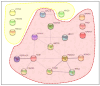
FBRSL1, together with FBRS and AUTS2 (Activator of Transcription and Developmental Regulator; OMIM 607270), constitutes a tripartite AUTS2 gene family. AUTS2 and FBRSL1 are evolutionarily more closely related to each other than to FBRS (Fibrosin 1; OMIM 608601). Despite its paralogous relation to AUTS2, FBRSL1's precise role remains unclear, though it likely shares functions in neurogenesis and transcriptional regulation. Herein, we report the clinical presentation with therapeutic approaches and the molecular etiology of a patient harboring a de novo truncating variant (c.371dupC) in FBRSL1, leading to a premature stop codon (p.Cys125Leufs*7). Our study extends previous knowledge by highlighting potential interactions and implications of this variant, alongside maternal and paternal duplications, for the patient's phenotype. Using sequence conservation data and in silico analysis of the truncated protein, we generated a predicted domain structure. Furthermore, our in silico analysis was extended by taking into account SNP array results. The extension of in silico analysis was performed due to the possibility that the coexistence of FBRSL1 truncating variant contemporary with maternal and paternal duplication could be a modifier of proband's phenotype and/or influence the novel syndrome clinical characteristics. FBRSL1 protein may be involved in neurodevelopment due to its homology with AUTS2, together with distinctive neuronal expression profiles, and thus should be considered as a potential modulation of clinical characteristics in a novel syndrome. Finally, considering that FBRSL1 is apparently involved in neurogenesis and in transcriptional regulatory networks that orchestrate gene expression, together with the observation that different genetic syndromes are associated with distinct genomic DNA methylation patterns, the specific episignature has been explored.
Keywords: FBRSL1 and AUTS2; neurodevelopment; polycomb complex.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ufartes R., Berger H., Till K., Sallinas G., Sturm M., Altmüller J., Nürnberg P., Thiele H., Funke R., Apeshiotis N., et al. De novo mutation in FBRSL1 cause a novel recognizable malformation and intellectual disability syndrome. Hum. Genet. 2020;139:1363–1379. doi: 10.1007/s00439-020-02175-x. - DOI - PMC - PubMed
-
- Baltz Alexander G., Munschauer M., Schwanhaüsser B., Vasile A., Murakawa Y., Schueler M., Youngs N., Penfold-Brown D., Drew K., Milek M., et al. The mRNA-Bound Proteome and Its Global Occupancy Profile on Protein-Coding Transcripts. Mol. Cell. 2012;46:674–690. doi: 10.1016/j.molcel.2012.05.021. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

