The Potential Links between lncRNAs and Drug Tolerance in Lung Adenocarcinoma
- PMID: 39062685
- PMCID: PMC11276205
- DOI: 10.3390/genes15070906
The Potential Links between lncRNAs and Drug Tolerance in Lung Adenocarcinoma
Abstract
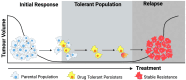
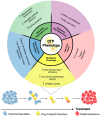
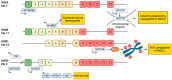
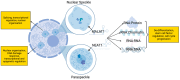
Lung cancer patients treated with targeted therapies frequently respond well but invariably relapse due to the development of drug resistance. Drug resistance is in part mediated by a subset of cancer cells termed "drug-tolerant persisters" (DTPs), which enter a dormant, slow-cycling state that enables them to survive drug exposure. DTPs also exhibit stem cell-like characteristics, broad epigenetic reprogramming, altered metabolism, and a mutagenic phenotype mediated by adaptive mutability. While several studies have characterised the transcriptional changes that lead to the altered phenotypes exhibited in DTPs, these studies have focused predominantly on protein coding changes. As long non-coding RNAs (lncRNAs) are also implicated in the phenotypes altered in DTPs, it is likely that they play a role in the biology of drug tolerance. In this review, we outline how lncRNAs may contribute to the key characteristics of DTPs, their potential roles in tolerance to targeted therapies, and the emergence of genetic resistance in lung adenocarcinoma.
Keywords: acquired drug resistance; drug tolerance; lncRNA; lung adenocarcinoma; targeted therapy.
Conflict of interest statement
Author Sarah Diermeier is the founder and a shareholder of the company Amaroq Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

