A Comparative Experimental and Computational Study on the Nature of the Pangolin-CoV and COVID-19 Omicron
- PMID: 39062780
- PMCID: PMC11277539
- DOI: 10.3390/ijms25147537
A Comparative Experimental and Computational Study on the Nature of the Pangolin-CoV and COVID-19 Omicron
Abstract
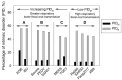
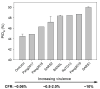
The relationship between pangolin-CoV and SARS-CoV-2 has been a subject of debate. Further evidence of a special relationship between the two viruses can be found by the fact that all known COVID-19 viruses have an abnormally hard outer shell (low M disorder, i.e., low content of intrinsically disordered residues in the membrane (M) protein) that so far has been found in CoVs associated with burrowing animals, such as rabbits and pangolins, in which transmission involves virus remaining in buried feces for a long time. While a hard outer shell is necessary for viral survival, a harder inner shell could also help. For this reason, the N disorder range of pangolin-CoVs, not bat-CoVs, more closely matches that of SARS-CoV-2, especially when Omicron is included. The low N disorder (i.e., low content of intrinsically disordered residues in the nucleocapsid (N) protein), first observed in pangolin-CoV-2017 and later in Omicron, is associated with attenuation according to the Shell-Disorder Model. Our experimental study revealed that pangolin-CoV-2017 and SARS-CoV-2 Omicron (XBB.1.16 subvariant) show similar attenuations with respect to viral growth and plaque formation. Subtle differences have been observed that are consistent with disorder-centric computational analysis.
Keywords: AI; COVID; XBB; artificial intelligence; attenuation; coronavirus; delta; intrinsic disorder; long COVID; membrane; nucleocapsid; nucleoprotein; omicron; pangolin; shell; vaccine; variant; virulence.
Conflict of interest statement
G.K.M.G. is the owner of Goh’s BioComputing, Singapore. He has written a book, “The Viral Shapeshifters: Strange Behaviors of HIV and Other Viruses”. The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Worobey M., Levy J.I., Malpica Serrano L., Crits-Christoph A., Pekar J.E., Goldstein S.A., Rasmussen A.L., Kraemer M.U.G., Newman C., Koopmans M.P.G., et al. The Huanan Seafood Wholesale Market in Wuhan was the early epicenter of the COVID-19 pandemic. Science. 2022;377:951–959. doi: 10.1126/science.abp8715. - DOI - PMC - PubMed
-
- Pekar J.E., Magee A., Parker E., Moshiri N., Izhikevich K., Havens J.L., Gangavarapu K., Malpica Serrano L.M., Crits-Christoph A., Matteson N.L., et al. The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2. Science. 2022;377:960–966. doi: 10.1126/science.abp8337. - DOI - PMC - PubMed
-
- WHO Coronavirus Disease (COVID-19) Pandemic. [(accessed on 8 June 2024)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

