Oxidative Metabolism as a Cause of Lipid Peroxidation in the Execution of Ferroptosis
- PMID: 39062787
- PMCID: PMC11276677
- DOI: 10.3390/ijms25147544
Oxidative Metabolism as a Cause of Lipid Peroxidation in the Execution of Ferroptosis
Abstract
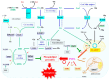
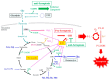
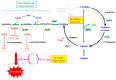
Ferroptosis is a type of nonapoptotic cell death that is characteristically caused by phospholipid peroxidation promoted by radical reactions involving iron. Researchers have identified many of the protein factors that are encoded by genes that promote ferroptosis. Glutathione peroxidase 4 (GPX4) is a key enzyme that protects phospholipids from peroxidation and suppresses ferroptosis in a glutathione-dependent manner. Thus, the dysregulation of genes involved in cysteine and/or glutathione metabolism is closely associated with ferroptosis. From the perspective of cell dynamics, actively proliferating cells are more prone to ferroptosis than quiescent cells, which suggests that radical species generated during oxygen-involved metabolism are responsible for lipid peroxidation. Herein, we discuss the initial events involved in ferroptosis that dominantly occur in the process of energy metabolism, in association with cysteine deficiency. Accordingly, dysregulation of the tricarboxylic acid cycle coupled with the respiratory chain in mitochondria are the main subjects here, and this suggests that mitochondria are the likely source of both radical electrons and free iron. Since not only carbohydrates, but also amino acids, especially glutamate, are major substrates for central metabolism, dealing with nitrogen derived from amino groups also contributes to lipid peroxidation and is a subject of this discussion.
Keywords: glycolysis; metabolic remodeling; tricarboxylic acid cycle; urea cycle.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

