Melanocortin-4 Receptor PLC Activation Is Modulated by an Interaction with the Monocarboxylate Transporter 8
- PMID: 39062808
- PMCID: PMC11277258
- DOI: 10.3390/ijms25147565
Melanocortin-4 Receptor PLC Activation Is Modulated by an Interaction with the Monocarboxylate Transporter 8
Abstract
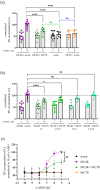
The melanocortin-4 receptor (MC4R) is a key player in the hypothalamic leptin-melanocortin pathway that regulates satiety and hunger. MC4R belongs to the G protein-coupled receptors (GPCRs), which are known to form heterodimers with other membrane proteins, potentially modulating receptor function or characteristics. Like MC4R, thyroid hormones (TH) are also essential for energy homeostasis control. TH transport across membranes is facilitated by the monocarboxylate transporter 8 (MCT8), which is also known to form heterodimers with GPCRs. Based on the finding in single-cell RNA-sequencing data that both proteins are simultaneously expressed in hypothalamic neurons, we investigated a putative interplay between MC4R and MCT8. We developed a novel staining protocol utilizing a fluorophore-labeled MC4R ligand and demonstrated a co-localization of MC4R and MCT8 in human brain tissue. Using in vitro assays such as BRET, IP1, and cAMP determination, we found that MCT8 modulates MC4R-mediated phospholipase C activation but not cAMP formation via a direct interaction, an effect that does not require a functional MCT8 as it was not altered by a specific MCT8 inhibitor. This suggests an extended functional spectrum of MCT8 as a GPCR signaling modulator and argues for the investigation of further GPCR-protein interactions with hitherto underrepresented physiological functions.
Keywords: BRET; MC4R; MCT8; fluorophore-labeled ligand; heterodimerization; protein–protein interaction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Clément K., Biebermann H., Farooqi I.S., van der Ploeg L., Wolters B., Poitou C., Puder L., Fiedorek F., Gottesdiener K., Kleinau G., et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 2018;24:551–555. doi: 10.1038/s41591-018-0015-9. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

