Loncastuximab Tesirine in the Treatment of Relapsed or Refractory Diffuse Large B-Cell Lymphoma
- PMID: 39062823
- PMCID: PMC11276998
- DOI: 10.3390/ijms25147580
Loncastuximab Tesirine in the Treatment of Relapsed or Refractory Diffuse Large B-Cell Lymphoma
Abstract
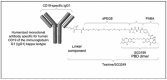
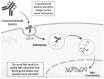
Currently, a significant percentage of patients with DLBCL are refractory or relapse after a first line of immunochemotherapy. Second relapses after autologous stem cell transplantation or chimeric antigen receptor T-cell therapies present few treatment options and do not yield good results. New molecules have entered the immunotherapy arsenal. Loncastuximab tesirine comprises a humanized anti-CD19 monoclonal conjugated antibody, which consists of an anti-CD19 antibody and cytotoxic alkylating agent, SG3199. Several studies have proven its efficacy in the treatment of refractory cases of DLBCL with a good safety profile, with the main adverse effects being neutropenia, thrombopenia, and liver enzyme involvement. In this review, we explain the mechanism of action of this molecule, the clinical data that have led to its acceptance by the FDA, and the new therapeutic options that are proposed in association with this drug.
Keywords: antibody–drug conjugate; diffuse large-B-cell lymphoma; immunotherapy; loncastuximab tesirine; refractory/relapse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Coiffier B., Lepage E., Brière J., Herbrecht R., Tilly H., Bouabdallah R., Morel P., Gisselbrecht C., Van Den Neste E., Salles G., et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. - DOI - PubMed
-
- Maurer M.J., Ghesquières H., Jais J.-P., Witzig T.E., Haioun C., Thompson C.A., Delarue R., Micallef I.N., Peyrade F., Macon W.R., et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J. Clin. Oncol. 2014;32:1066–1073. doi: 10.1200/JCO.2013.51.5866. - DOI - PMC - PubMed
-
- Cheson B.D., Horning S.J., Coiffier B., Shipp M.A., Fisher R.I., Connors J.M., Lister T.A., Vose J., Grillo-López A., Hagenbeek A., et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J. Clin. Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. - DOI - PubMed
-
- Crump M., Neelapu S.S., Farooq U., Van Den Neste E., Kuruvilla J., Westin J., Link B.K., Hay A., Cerhan J.R., Zhu L., et al. Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood. 2017;130:1800–1808. doi: 10.1182/blood-2017-03-769620. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources