Structure Engineering of Ni/SiO2 Vegetable Oil Hydrogenation Catalyst via CeO2
- PMID: 39062829
- PMCID: PMC11276988
- DOI: 10.3390/ijms25147585
Structure Engineering of Ni/SiO2 Vegetable Oil Hydrogenation Catalyst via CeO2
Abstract
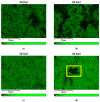
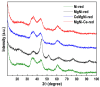
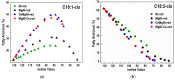
Inspired by our finding that metallic Ni particles could be uniformly distributed on a reduced CeO2 surface and stabilized on Ce3+ sites, we suppose a possible improvement in the activity and selectivity of the MgNi/SiO2 vegetable oil hydrogenation catalyst by increasing the surface metal Ni availability via modification by ceria. The proposed approach involved the addition of a CeO2 modifier to the SiO2 carrier and as a catalyst component. Evaluation of the structure, reducibility, and surface and electronic states of the CeO2-doped MgNi/SiO2 catalyst was performed by means of the Powder X-ray diffraction (PXRD), Scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS), and X-ray photoelectron spectroscopy (XPS) combined with High-resolution transmission electron microscopy (HRTEM), Temperature-programmed reduction with hydrogen (H2-TPR), and H2-chemisortion techniques. So far, no studies related to this approach of designing Ni/SiO2 catalysts for the partial hydrogenation of vegetable oil have been reported. The added ceria impact was elucidated by comparing fatty acid compositions obtained by the catalysts at an iodine value of 80. In summary, tuning the hydrogenation performance of Ni-based catalysts can be achieved by structural reconstruction using 1 wt.% CeO2. The introduction mode changed the selectivity towards C18:1-cis and C18:0 fatty acids by applying ceria as a carrier modifier, while hydrogenation activity was improved upon ceria operation as the catalyst dopant.
Keywords: CeO2 doping effect; HRTEM; Ni/SiO2 catalysts; SEM-EDS; fatty acids composition; partial hydrogenation of sunflower oil.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Figures






References
-
- Coenen J. Catalytic Hydrogenation of Fatty Oils. Ind. Eng. Chem. Fundam. 1986;25:43–52. doi: 10.1021/i100021a006. - DOI
-
- Patterson H.B.W. Hydrogenation of Fats and Oils: Theory and Practice. AOCS Press; Champaign, IL, USA: 1994. p. 267.
-
- Veldsink J.W., Bouma M.J., Schöön N.H., Beenackers A.A.C.M. Heterogeneous Hydrogenation of Vegetable Oils: A Literature Review. Catal. Rev. 1997;39:253–318. doi: 10.1080/01614949709353778. - DOI
-
- Lee K.-W., Mei B.X., Bo Q., Kim Y.-W., Chung K.-W., Han Y. Catalytic Selective Hydrogenation of Soybean Oil for Industrial Intermediates. J. Ind. Eng. Chem. 2007;13:530–536.
-
- Balakos M.W., Hernandez E.E. Catalyst characteristics and performance in edible oil hydrogenation. Catal. Today. 1997;35:415–425. doi: 10.1016/S0920-5861(96)00212-X. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

