Transcriptomic Profile of Breast Tissue of Premenopausal Women Following Treatment with Progesterone Receptor Modulator: Secondary Outcomes of a Randomized Controlled Trial
- PMID: 39062832
- PMCID: PMC11277027
- DOI: 10.3390/ijms25147590
Transcriptomic Profile of Breast Tissue of Premenopausal Women Following Treatment with Progesterone Receptor Modulator: Secondary Outcomes of a Randomized Controlled Trial
Abstract
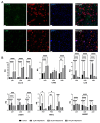
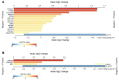
Progesterone receptor antagonism is gaining attention due to progesterone's recognized role as a major mitogen in breast tissue. Limited but promising data suggest the potential efficacy of antiprogestins in breast cancer prevention. The present study presents secondary outcomes from a randomized controlled trial and examines changes in breast mRNA expression following mifepristone treatment in healthy premenopausal women. We analyzed 32 paired breast biopsies from 16 women at baseline and after two months of mifepristone treatment. In total, 27 differentially expressed genes were identified, with enriched biological functions related to extracellular matrix remodeling. Notably, the altered gene signature induced by mifepristone in vivo was rather similar to the in vitro signature. Furthermore, this gene expression signature was linked to breast carcinogenesis and notably linked with progesterone receptor expression status in breast cancer, as validated in The Cancer Genome Atlas dataset using the R2 platform. The present study is the first to explore the breast transcriptome following mifepristone treatment in normal breast tissue in vivo, enhancing the understanding of progesterone receptor antagonism and its potential protective effect against breast cancer.
Keywords: breast cancer; mifepristone; progesterone receptor antagonist; progesterone signaling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Coelingh Bennink H.J.T., Schultz I.J., Schmidt M., Jordan V.C., Briggs P., Egberts J.F.M., Gemzell-Danielsson K., Kiesel L., Kluivers K., Krijgh J., et al. Progesterone from ovulatory menstrual cycles is an important cause of breast cancer. Breast Cancer Res. 2023;25:60. doi: 10.1186/s13058-023-01661-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

