Potential for Drug Repositioning of Midazolam as an Inhibitor of Inflammatory Bone Resorption
- PMID: 39062893
- PMCID: PMC11277201
- DOI: 10.3390/ijms25147651
Potential for Drug Repositioning of Midazolam as an Inhibitor of Inflammatory Bone Resorption
Abstract
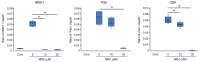
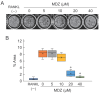
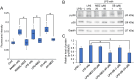
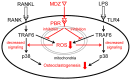
Drug repositioning is a method for exploring new effects of existing drugs, the safety and pharmacokinetics of which have been confirmed in humans. Here, we demonstrate the potential drug repositioning of midazolam (MDZ), which is used for intravenous sedation, as an inhibitor of inflammatory bone resorption. We cultured a mouse macrophage-like cell line with or without MDZ and evaluated its effects on the induction of differentiation of these cells into osteoclasts. For in vivo investigations, we administered lipopolysaccharide (LPS) together with MDZ (LPS+MDZ) to the parietal region of mice and evaluated the results based on the percentage of bone resorption and calvaria volume. Furthermore, we examined the effects of MDZ on the production of reactive oxygen species (ROS) in cells and on its signaling pathway. MDZ inhibited osteoclast differentiation and bone resorption activity. In animal studies, the LPS+MDZ group showed a decreasing trend associated with the rate of bone resorption. In addition, the bone matrix volume in the LPS+MDZ group was slightly higher than in the LPS only group. MDZ inhibited osteoclast differentiation by decreasing ROS production and thereby negatively regulating the p38 mitogen-activated protein kinase pathway. Thus, we propose that MDZ could potentially be used for treating inflammatory bone resorption, for example, in periodontal disease.
Keywords: bone resorption; drug repositioning; inflammation; midazolam; reactive oxygen species; signal transduction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Aminothiazoles inhibit osteoclastogenesis and PGE2 production in LPS-stimulated co-cultures of periodontal ligament and RAW 264.7 cells, and RANKL-mediated osteoclastogenesis and bone resorption in PBMCs.J Cell Mol Med. 2019 Feb;23(2):1152-1163. doi: 10.1111/jcmm.14015. Epub 2018 Dec 1. J Cell Mol Med. 2019. PMID: 30506812 Free PMC article.
-
6,7,4'-Trihydroxyflavone inhibits osteoclast formation and bone resorption in vitro and in vivo.Phytother Res. 2019 Nov;33(11):2948-2959. doi: 10.1002/ptr.6468. Epub 2019 Sep 3. Phytother Res. 2019. PMID: 31478281
-
Quercetin triggers apoptosis of lipopolysaccharide (LPS)-induced osteoclasts and inhibits bone resorption in RAW264.7 cells.Cell Physiol Biochem. 2012;30(1):123-36. doi: 10.1159/000339052. Epub 2012 Jan 8. Cell Physiol Biochem. 2012. PMID: 22759961
-
The protective effect of WKYMVm peptide on inflammatory osteolysis through regulating NF-κB and CD9/gp130/STAT3 signalling pathway.J Cell Mol Med. 2020 Jan;24(2):1893-1905. doi: 10.1111/jcmm.14885. Epub 2019 Dec 14. J Cell Mol Med. 2020. PMID: 31837208 Free PMC article.
-
Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases.Int J Mol Sci. 2019 Jul 22;20(14):3576. doi: 10.3390/ijms20143576. Int J Mol Sci. 2019. PMID: 31336616 Free PMC article. Review.
Cited by
-
Diabetes and periodontitis: the role of a high-glucose microenvironment in periodontal tissue cells and corresponding therapeutic strategies.Stem Cell Res Ther. 2025 Jul 15;16(1):366. doi: 10.1186/s13287-025-04441-z. Stem Cell Res Ther. 2025. PMID: 40660319 Free PMC article. Review.
-
Substrate-Mediated Regulation of Src Expression Drives Osteoclastogenesis Divergence.Genes (Basel). 2024 Sep 18;15(9):1217. doi: 10.3390/genes15091217. Genes (Basel). 2024. PMID: 39336808 Free PMC article.
References
-
- Davide G., Nicolò S., Diana T., Fabiola R., Sara B., Serena B., Maurizio P., Giuseppe V. Regenerative Potential of Platelet—Rich Fibrin in Maxillary Sinus Floor Lift Techniques: A Systematic Review. J. Biol. Regul. Homeost. Agents. 2023;37:2357–2369. doi: 10.23812/j.biol.regul.homeost.agents.20233705.232. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

